Molecular Mechanisms Of Pd
PD is a multifactorial disease , where both genetic and non-genetic, such as environmental factors, are involved . The most salient mechanisms involved in the development of PD include the accumulation of misfolded proteins aggregates, failure of protein clearance pathways, mitochondrial damage, oxidative stress, excitotoxicity, neuroinflammation, and genetic mutations .
Fig. 3
Schematic diagram showing the involvement of different factors and signaling pathways for degeneration of DA-neurons in PD
Changes In Cell Metabolism
The third major proposed cause of cell death in Parkinson’s disease involves the energy-generating mitochondrion organelle. In Parkinson’s disease, mitochondrial function is disrupted, inhibiting energy production and resulting in death.
The mechanism behind mitochondrial dysfunction in Parkinson’s disease is hypothesized to be the PINK1 and Parkin complex, having been shown to drive autophagy of mitochondria . PINK1 is a protein normally transported into the mitochondrion, but can also accumulate on the surface of impaired mitochondria. Accumulated PINK1 then recruits Parkin; Parkin initiates the break down of dysfunctional mitochondria, a mechanism that acts as a “quality control” In Parkinson’s disease, the genes coding PINK1 and Parkin are thought to be mutated, therefore preventing the breakdown of impaired mitochondria, causing abnormal function and morphology of mitochondria and eventually cell death Mitochondrial DNA mutations have also been shown to accumulate with age indicating that susceptibility to this mechanism of neuronal death increases with age.
Pd Pathophysiology And Diagnosis
Currently, the molecular mechanisms underlying the pathophysiology of PD remain largely unknown. To date, -Syn accumulation, mitochondrial dysfunction, oxidative stress, and excitotoxicity are thought to play crucial roles in PD pathophysiology .
The accumulation of -Syn progresses predictably throughout the brain, typically known as the Braak stage. The lesions initially begin from the dorsal motor nucleus of the glossopharyngeal and vagal nerves and the anterior olfactory nucleus. They then ascend to the brain stem and finally to the neocortex . The reasons why -Syn, an intrinsically disordered protein, misfolds and deposits in the brain are still obscure. The currently accepted explanation is imbalance between its synthesis and clearance . SNCA gene duplication, triplication, or mutation aggravates the accumulation of misfolded -Syn . Moreover, the collapse of any pathways involved in -Syn clearance and degradation results in the accumulation of -Syn . For instance, disruption of the lysosomal degradation pathway promotes the formation of -Syn inclusions in cells .
Don’t Miss: Are Intention Tremors Common In Parkinson’s
What Is Parkinsons Disease
Parkinsons disease is a progressive neurological disorder, and is classied as a Movement Disorder, as it primarily affects movement. It is variable in its progression, i.e. some people progress more slowly than others, and the symptoms can be effectively controlled with medication for many years. Parkinsons disease is caused by a loss of a chemical called dopamine. We all lose some of this chemical as we get older, however, it is only when we have lost about 80% of our dopamine we start to have symptoms. So people with Parkinsons have lost this chemical at a faster rate than others.
Although Parkinsons is a movement disorder, there are both motor and non-motor symptoms associated with it.
Parkinsons can be difcult to diagnose initially, it may take up to 2-3 visits before a conclusive diagnosis is made.
What is Parkinsonism?
Parkinsonism is an umbrella term that describes conditions that feature the main symptoms of Parkinsons . About 85% of people with Parkinsonism have Parkinsons. The other 15% have other rarer conditions such as Drug Induced Parkinsons, Progressive Supranuclear Palsy , Multiple System Atrophy and Parkinsonism secondary to other medical problems, such as after a stroke.
At What Age Does Parkinsons Disease Occur?
How Common is Parkinsons Disease?
What Causes Parkinsons Disease?
Can Parkinsons Disease be Inherited?
Signs and Symptoms
The motor symptoms are tremor, stiffness, slowness, stooped posture and gait disturbance/ impaired balance.
What Is Parkinson’s Disease
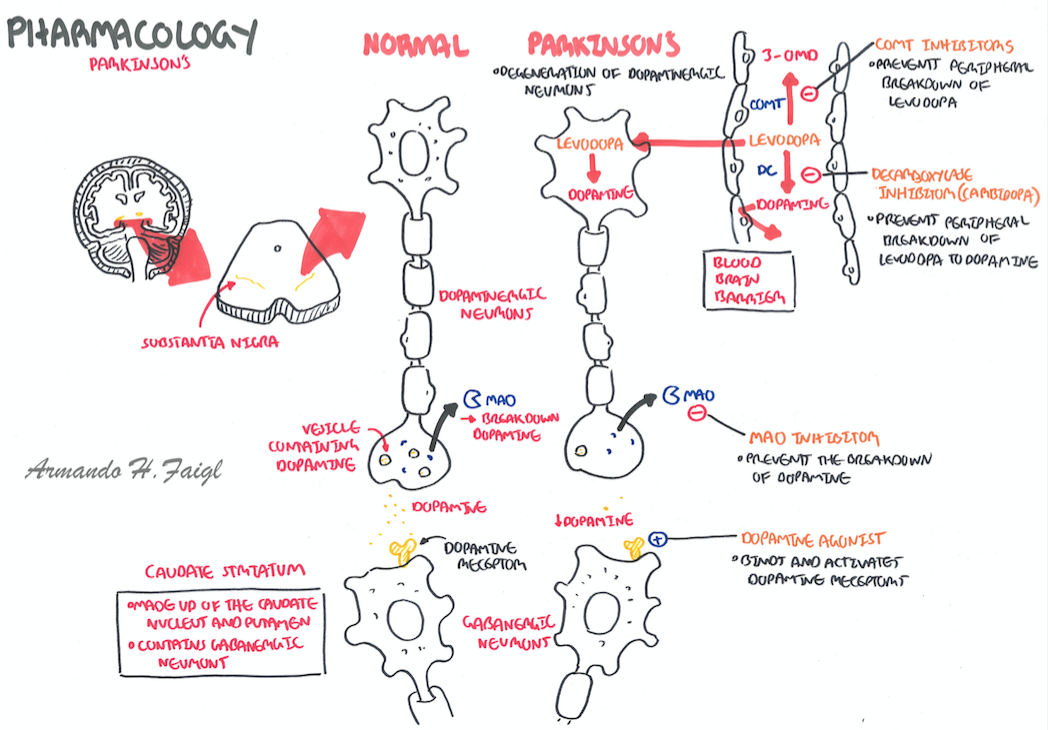
Parkinsons disease is the deterioration of brain nerves that control movement. The symptoms of Parkinsons disease have a slow onset and get worse over time. You may experience a gradual onset of symptoms, or notice several changes all at once.
Perhaps the most well-known symptom of Parkinsons disease is the development of a tremor. You may notice that your fingers, hands, or chin shake uncontrollably. Other symptoms include:
- Change in handwriting specifically smaller handwriting
- Changes in your tone of voice specifically speaking more quietly
- Lack of facial expressions
- Dizziness and fainting
- Beginning to walk with a hunched back
It is important to keep in mind that medications and other medical conditions can cause symptoms similar to those listed above. But, if you are experiencing a combination of these symptoms, it may be a sign of Parkinsons disease.
While there is not currently a cure for Parkinsons disease, many treatment options are available that can help ease your symptoms. Treatments may include medicine, therapy, and even surgery. Each case of Parkinsons disease is unique, and your treatment plan should be, too.
Also Check: Does Parkinson’s Affect Your Mind
A Potential Role For T Cells In Parkinson’s Disease
In the last decade there has been mounting evidence of a role for T cells in the pathogenesis of PD . Despite their important role as part of the adaptive immune system there is little evidence of the involvement of B cells in PD. Brochard et al., identified both CD8+ and CD4+ T cells but not B cells or natural killer cells in the post-mortem brain tissue of PD patients . The presence of both CD8+ and CD4+ T cells was also evident in the MPTP and -syn overexpressing mouse models of PD . However, Theodore et al., observed both infiltrating B and T cells following injection with -syn overexpressing adeno-associated viral vector into the SN of mice . The differences with respect to the possible role of B cells in these studies may be attributed to the study model and human PD tissue vs. animal model, however more research is required before a definitive conclusion can be drawn.
Table 3. Evidence for the role of T lymphocytes in Parkinson’s disease.
The Role of MHC and Antigen Presentation in Parkinson’s Disease
Mitochondrial Antigen Presentation in Parkinson’s Disease
Th1 Cells in Parkinson’s Disease
Th17 Cells in Parkinson’s Disease
Th2 Cells in Parkinson’s Disease
T Cell Regulation in Parkinson’s Disease
Dysfunctional Protein Clearance Systems
There are two central protein clearance systems within cells responsible for the removal of dysfunctional proteins: the ubiquitin-proteasome system and the autophagy-lysosome pathway. The UPS is primarily responsible for breaking down abnormal proteins, and it does so by tagging them with ubiquitin and transporting them to the proteasome for degradation. The autophagy-lysosome pathway is divided into three constituents: macroautophagy, microautophagy, and chaperone-mediated autophagy . Briefly, in macroautophagy, intracellular components, including cytosolic proteins, are engulfed by the autophagosome, which then fuses with the lysosome, leading to the breakdown of its contents. On the other hand, in microautophagy, the lysosome alone engulfs and destroys cytoplasmic components. CMA is a more selective process, whereby molecular chaperones target specific proteins and transport them to the lysosome for degradation . Monomeric -synuclein is generally cleared by both the UPS and the autophagy-lysosome pathway , and damage in either of their machineries is implicated in the pathogenesis of PD by contributing to the accumulation of defective proteins, in particular soluble misfolded -synuclein .
Read Also: How To Help People With Parkinson’s Disease
Selective Vulnerability Of The Nigrostriatal Dopamine Neuron
In PD, dopamine neurons within the substantia nigra are considered a selectively susceptible population of cells, whereas the adjacent dopamine neurons of the ventral tegmental area are much more resistant to degeneration. The vulnerability of the nigrostriatal neurons is due to several factors, including their unique anatomy, physiology, bioenergetic profile, and neurochemistry . First, in rat brain, the length of the axon arbor of a single nigrostriatal neuron is up to 80 cm; in humans, this is estimated to be 4 m! In the rat, a single nigrostriatal neuron makes 100000240000 synapses in the striatum. In humans, it is estimated that a nigrostriatal neuron makes 10000002400000 synapses. By contrast, the VTA neuron makes about 10-fold fewer synapses and therefore has a much lower bioenergetic demand. Further compounding the bioenergetic demand of the nigrostriatal neuron is the fact that their axons are unmyelinated. Thus, propagation of each action potential and subsequent repolarization requires much more energy than if the fibers were myelinated.
Validated Tools For Grading Non
There are now several validated tools available that empower patients to report their symptoms and allow clinicians to measure and grade the holistic burden. The available tools that address NMS include the NMS Questionnaire, the NMS Scale, as well as the IPMDS unified Parkinson’s disease rating scale part 1. In clinical practice, it is recommended to apply these tools together with a standard motor and clinimetric assessment, such as Hoehn and Yahr,48 UPDRS part 24 as well as clinical impression of severity index . As a consequence, the progression of the condition and the response to treatment can be measured in a structured manner, allowing for both NMS and motor symptoms to be addressed without the risk of remaining undeclared.
A proposed algorithm for holistic motor and non-motor assessment of patients with Parkinsons disease in clinical practice. CISI-PD = clinical impression of severity index- Parkinsons disease; MDS = movement disorders society; NMS = Non-motor symptoms; UPDRS = Unified Parkinsons disease rating scale.
Also Check: What Type Of Exercise Is Best For Parkinson’s
Neuroinflammation Involved In Pd
A cascade of events are involved in neuroinflammation processes in PD, including activation of microglia and an increase secretion of cytokines . For example, researchers have found strong links between pro-inflammatory cytokines and degeneration of DA neurons, following sub-chronic administration of MPTP in animals . Several clinical studies have shown that the level of inflammatory enzymes, such as cyclo-oxygenase-2 , is increased several times in DA-neurons of the postmortem PD brain and in a mouse models of PD .
Fig. 11
Mechanism of neuroinflammation in PD. T-lymphocytes and complementary systems can activate microglia to secrete several cytokines, which causes DA-neuronal injury. Similarly, aggregated SNCA can also activate astrocytes, which causes oxidative stress, leading to neuronal injury
Prion hypothesis
Pd Caused By Impairment Of Protein Degradation Pathways
Molecular chaperones . The molecular chaperone, is one of the most efficient, highly conserved cellular defense mechanisms involved in protein folding, refolding of partially misfolded proteins, and protein degradation . Major HSPs involved in PD are HSP 26, 40, 60, 70, 90 and 100. Some of the HSPs are localized in synapses and axons, and their levels are down-regulated in PD as well as other neurodegenerative diseases . Importantly, HSPs can bind to aggregated SNCA or tau oligomers or pre-fibrillar structures, and interfere by forming low MW soluble oligomers or higher order insoluble structures which reduce their toxicity . HSPs also play pivotal roles in the regulation and precise functioning of ubiquitin proteasome and the autophagy-lysosomal pathways .
In drosophila and yeast models of PD, HSP70 co-expression prevents DA cell death by decreasing the SNCA toxicity , whereas mutations of ATPase domain in HSP70 increase toxicity . Similarly, over-expression of HSP70 decreases MPTP- or rotenone-induced neurotoxicity in rat brain slices and also in cultured SK-N-SH or PC12 cells . Furthermore, a reduction of total and detergent-insoluble fractions of misfolded SNCA aggregates were observed in an in vitro model of PD, which co-express different yeast HSPs , suggesting molecular chaperones become dysregulated in PD.
Fig. 5Fig. 6
You May Like: How Does A Neurologist Diagnose Parkinson’s
Association Between Mapt Gene And Pd
As the popularity of GWAS technology has grown, numerous studies have been performed to explore risk loci for PD et al. 2014). Thus far, at least 41 risk loci have been identified to be associated with PD , among which, MAPT is one of the most studied risk genes. Two extended haplotypes, H1 and H2, differ in orientation and do not recombine, covering the entire MAPT gene . Variants in MAPT can increase the risk of PD and influence the progression and clinical manifestations of the disease. A full sequencing and haplotype analysis of MAPT in PD showed that the H1 haplotype is associated with an increased risk of PD but that the H2 haplotype has protective effects . The pathologic effects of the H1 haplotype seem to vary in different populations. For example, the results of GWAS in Europe and America show a significant association . In contrast, the results of studies in Asia are not consistent . Such a lack of consensus in investigations among different populations likely stems from the effects of population structure and population-specific environmental interactions, and the different results of studies in Asia may be caused by clinical heterogeneity and variable reliability in methodological issues. Hence, more studies are needed to explore the influences of MAPT on PD onset in the Asian population.
Changes In Sensory Response Patterns And Changes In Task
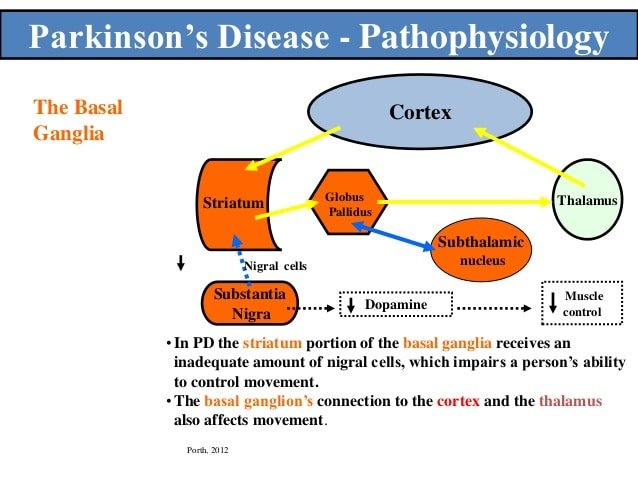
Under normal conditions, many neurons within the motor territory of each of the basal ganglia structures respond to proprioceptive input. Appropriate modulation of basal ganglia activity through such inputs may be important to gate and restrict cortical activities related to specific movements or other activities. Sensory responses in the striatum can be assumed to be the result of direct cortical inputs to this structure, while proprioceptive responses in the extrastriatal basal ganglia are, at least in part, due to inputs reaching the STN via the cortico-subthalamic projection . Pallidal recordings in MPTP-treated animals showed a reduction in the specificity of such responses , and an increase in the proportion of neurons with excitatory responses . In addition, rodent studies showed that the normal arrangement of striatal neurons into clusters that respond to sensory inputs appears to be fragmented in the dopamine-depleted state . These sensory changes may be due to abnormal basal ganglia processing, or may reflect abnormal cortical inputs to the basal ganglia. Through disruption of cortico-subcortical feedback mechanisms that control the extent and speed of movement, they may contribute to abnormal scaling of movements and bradykinesia in Parkinsons disease .
Also Check: What Country Has The Most Parkinson’s Disease
Changes In Cortical Activity
Early 2-deoxyglucose studies suggested that cortical activation is globally reduced in MPTP-treated monkeys . Later studies using positron emission tomography and functional magnetic resonance imaging in parkinsonian patients documented changes in activity both at rest and during the performance of motor or cognitive tasks. The PET studies demonstrated that cortical activation in motor tasks is reduced in parkinsonism, specifically in the supplementary motor area and in the anterior cingulate cortex .
Besides the obvious motor impairments, parkinsonian patients also show abnormalities in cognitive tasks, perhaps associated with the loss of dopamine in non-motor portions of the striatum . PET studies in such patients have suggested that at least some of the cognitive deficits could be explained by abnormal activation of non-motor areas of the basal ganglia .
A small number of electrophysiologic studies have examined changes in cortical activities in MPTP-treated monkeys. These studies found that the activation of motor cortex or the supplementary motor area is reduced in these animals , and that the synchrony between neurons is increased . EEG studies in patients have shown that parkinsonism may be associated with abnormal beta-band synchronization of cortical networks, , and a failure to modulate frontal and central beta-band activity with movement .
Microglia And Their Role In Parkinson’s Disease
Microglia are the resident immune cells of the CNS. They originate in the yolk sac where they develop from early myeloid precursor cells. During embryonic development, primitive microglia migrate into the developing neural tube where they proliferate and populate the CNS . Due to the BBB, microglia lead a relatively sheltered existence compared to peripheral macrophages, although their functions remain the same. Their role is to continuously survey the microenvironment and respond to both physiological and pathological changes. In their capacity as the first line of defence in the CNS, they identify and remove unwanted material such as cellular debris.
Read Also: How Long Does It Take To Die From Parkinson’s Disease
How Do You Know You Have Parkinsons Disease
There is no definitive way to diagnose Parkinsons disease. Your doctor will ask questions about the onset of your symptoms and assess your movement to make referrals to specialists who can make a formal diagnosis.
You can expect to see a neurologist who can complete a neurologic examination. This may include brain imaging, an MRI, or a PET scan to see activity in the area of the brain typically affected by Parkinsons disease.
Your doctor may also refer you to a movement disorder specialist. Seeing subspecialists is very important to avoid being misdiagnosed. Highly trained specialists can provide their expertise in specific areas of medicine where a precise diagnosis isnt possible from blood work or another definitive test.
The Genetics Of Parkinsons
A 2020 study including 1,676 people with Parkinsons in mainland China suggested that genes play a role in the development of the condition. An estimated 10 to 15 percent of people with Parkinsons have a family history of the condition.
In fact, a number of specific genes have been linked to the development of Parkinsons.
How do genetics factor into Parkinsons in some families? According to Genetics Home Reference, one possible way is through the mutation of genes responsible for producing dopamine and certain proteins essential for brain function.
Also Check: Can Parkinson’s Disease Cause Personality Changes
Parkinson’s Disease Pathology Aetiology And Diagnosis
Published online by Cambridge University Press: 08 June 2012
- Department of Medicine for the Elderly/Movement Disorders Clinic, Southern General Hospital, Victoria Infirmary, Glasgow, UK
- David A Stewart
- Department of Medicine for the Elderly/Movement Disorders Clinic, Southern General Hospital, Victoria Infirmary, Glasgow, UK
- Corresponding
Experimental Evidence Supporting The Role Of Tau In Pd Pathophysiology
Various studies have employed transgenic PD mouse lines in which Tau is manipulated, reduced, or eliminated to explore the potential molecular pathways through which Tau may contribute to the pathophysiology of PD . Nevertheless, no consistent conclusion can be drawn thus far. The differences in the results of those studies may be due to intermodel variability. Some studies have been performed to explore changes in Tau protein in PD models. Wills and coworkers found increased hyperphosphorylated Tau in the striatum of adult A53T -synuclein transgenic mice that colocalized with -Syn, which was aggregated and accumulated in inclusion bodies . Further studies have shown that overexpression or mutation of -Syn may increase the phosphorylation of Tau by promoting expression of GSK-3, a primary kinase known to phosphorylate Tau at multiple sites . Bardai et al. reported that the leucine-rich repeat kinase 2 protein and mutations in the gene encoding it are the most common causes of familial PD and promote Tau neurotoxicity through dysregulation of actin and mitochondrial dynamics . These studies suggest that Tau and -Syn can promote each others pathological changes to form a vicious cycle, ultimately promoting the occurrence and development of PD.
Recommended Reading: Is Parkinson’s Disease Deadly
The Impact Of Parkinson’s Disease Genes On Microglial Function
PD-associated genes are expressed by microglia, not just neurons and astrocytes. The products of these gene mutations are thought to affect the functioning of these cells , and can exacerbate microgliosis . The dominant PD-risk genes SNCA and LRRK2 promote neuroinflammation via activation of microglia and inflammatory signalling pathways such as NF-B. Besides cell activation, PD-associated genes also disrupt other microglial processes including mitochondrial respiration and autophagy . Since autophagy is involved in regulating microglia inflammatory status , its disruption has been reported to play a critical role in inflammation and may also affect some of the key functions of microglia including phagocytosis . Furthermore, autophagy failure has been shown to promote intercellular propagation of -syn which in turn drives microglial activation and neuroinflammation. Mitochondrial dysfunction is another pathological feature in PD, as such the mitochondrial toxin MPTP, is commonly used to induce PD pathology in rodents in order to model disease pathology. Furthermore, pesticides paraquat and rotenone can also be used to induce parkinsonism via disruption of the respiratory chain in mitochondria . A number of genes associated with increased PD risk are linked to mitochondrial homeostasis. Among these are SNCA, PARK2, PINK1, PARK7, and LRRK2 .
Table 2. The role of microglia and Parkinson’s disease risk genes in Parkinson’s disease pathology.
DJ-1/PARK7
SNCA
LRRK2
The Five Environmental Causes Of Parkinsons Disease

Parkinsons disease is a brain disorder that develops and becomes worse over time. The typical symptoms of the disease include tremor, stiffness, slowness of movement, and balance problems. These symptoms develop when the brain lost its ability to produce a sufficient amount of dopamine, a neurotransmitter responsible for controlled body movement.
Researchers have identified a variety of environmental factors that are linked to Parkinsons disease. Some of these factors may directly cause the disease symptoms, others may increase the risk of developing it.
Here are the 5 main environmental factors that are linked to Parkinsons disease development.
Recommended Reading: Can A Plant Based Diet Help Parkinson’s
Do Electrophysiologic Abnormalities In The Basal Ganglia
There is little doubt that moderate or severe forms of parkinsonism are associated with increased bursting, oscillatory activity, changes in interneuronal synchrony, changes in the processing of sensory information, and perhaps changes in firing rates. The immediate beneficial effects of focal lesions or DBS in the basal ganglia suggests that such changes may contribute to the development of the behavioral manifestations of the disease. However, the importance of specific electrophysiologic features in basal ganglia, thalamic or cortical activity for the development of the behavioral signs of parkinsonism has not been fully worked out yet.
While the aforementioned studies in moderately or severely parkinsonian patients or animals suggest that synchronous oscillatory activity is associated with parkinsonism, these studies do not prove causality. Thus, it is not known whether 10- or 20 Hz stimulation actually induces low-frequency oscillations in the basal ganglia-thalamocortical network, and, if so, whether the oscillation act through disruption of information processing, or through associated changes in transmitter levels or other biochemical alterations. It is also not known whether levodopa treatments or STN-DBS reduce akinesia because the reduce beta-band activity, or whether the reduction of these oscillations is simply correlated with the beneficial motor effects.
Dopamine Loss In The Striatum
In Parkinsons disease, the degeneration of dopaminergic SNc neurons and their projections to the striatum is a slowly evolving process that may take decades to develop. SNc projections to the putamen degenerate earlier than projections to associative or limbic portions of the striatum. Corresponding to this time course of degeneration, the motor symptoms and signs of Parkinsons disease develop before the non-motor signs.
Recognizable motor or non-motor signs appear only after substantial degeneration of the nigrostriatal neurons , testament to the remarkable compensatory capacity within the dopaminergic system, or in the circuits it modulates.
Dopamine loss in the basal ganglia triggers prominent secondary morphological changes. One change that may have pathophysiologic significance is the reduction of the density of dendritic spines on MSNs, particularly in the putamen , which may greatly alter corticostriatal transmission. Recent studies have suggested that MSNs with D2 receptors may be preferentially affected by the spine loss, and that the loss of spines may involve the dysregulation of calcium channels .
Recommended Reading: What Brain Structure Is Affected By Parkinson’s
Changes In Thalamic Activity
In this section, we will consider parkinsonism-related changes in VA/VL separately from those occurring in CM/Pf, because these thalamic nuclear groups appear to have different physiologic functions. VA/VL are part of the basal ganglia-thalamocortical circuitry, while CM/Pf participate in circuits by which basal ganglia output is fed back into the putamen.
With regard to the CM/Pf nuclei, there is indirect evidence that GABAergic basal ganglia output to these nuclei is increased in parkinsonism, in line with predictions of the rate model of parkinsonism . Thus, GABA-A receptor subunit expression in Pf is decreased in dopamine-depleted rats , and a decrease in average Pf firing rates has been documented in such animals . There is also evidence that MPTP-exposure in monkeys leads to changes in glucose utilization in CM/Pf , suggesting changes in synaptic or neuronal activity. The significance of activity changes in CM/Pf in the development of parkinsonism has not been explored in detail. According to the circuitry model in , parkinsonism may result in a reduction of CM/Pf activity which may, secondarily, result in reduced driving of direct pathway MSNs , and worsening of parkinsonism. This pathophysiologic scheme may be too simplistic, however, because the effects of altered patterns of basal ganglia input to CM/Pf, and of CM outputs acting on striatal interneurons are not taken into account.
