Dopamine Hypothesis Of Depression
Dopamine is produced in the substantia nigra pars compacta in the midbrain. Dopaminergic projections in both the mesocortical and the mesolimbic systems are known to be disturbed by stress . Dopaminergic pathways are part of the reward system and the effects of chronic stress on reward perception that lead to depression can occur because of the interaction between the dopaminergic system and the HPA axis and between the dopaminergic system and the serotonergic system . Studies have demonstrated that early psychological stress that activates the HPA axis, exacerbates DA depletion and is associated with a decrease in DA synthesis in the brain . Auffret et al. and Leentjens, have shown that symptoms of depression can be improved by administration of DA agonists highlighting the possibility of antidepressant drugs to have an affinity to DA receptors. Since DA depletion may accompany depression, some antidepressant drugs may act on both dopaminergic and serotonergic systems to exert their antidepressant effect . Therefore, DA deficiency resulting from early life stress may in some instances predispose an individual to depression and eventually to neurodegenerative diseases such as PD.
What Are The Symptoms
Symptoms of PD vary from person to person, as does the rate of progression. A person who has Parkinson’s may experience some of these more common “hallmark” symptoms:
- Bradykinesia – slowness of movement, impaired dexterity, decreased blinking, drooling, expressionless face.
- Tremor at rest – involuntary shaking that decreases with purposeful movement. Typically starts on one side of the body, usually the hand.
- Rigidity – stiffness caused by involuntary increase in muscle tone.
- Postural instability – sense of imbalance. Patients often compensate by lowering their center of gravity, which results in a stooped posture.
Other symptoms that may or may not occur:
Freezing or being stuck in place Shuffling gait or dragging of one foot Stooped posture Cognitive impairment
What Treatments Are Available
Many Parkinson’s patients enjoy an active lifestyle and a normal life expectancy. Maintaining a healthy lifestyle by eating a balanced diet and staying physically active contributes to overall health and well-being. Parkinson’s disease can be managed with self-care, medication, and surgery.
Self careExercise is as important as medication in the treatment of PD. It helps maintain flexibility and improves balance and range of motion. Patients may want to join a support group and continue enjoyable activities to improve their quality of life. Equally important is the health and well being of the family and caregivers who are also coping with PD. For additional pointers, see Coping With Parkinsons Disease.
These are some practical tips patients can use:
Medications There are several types of medications used to manage Parkinson’s. These medications may be used alone or in combination with each other, depending if your symptoms are mild or advanced.
After a time on medication, patients may notice that each dose wears off before the next dose can be taken or erratic fluctuations in dose effect . Anti-Parkinsons drugs can cause dyskinesia, which are involuntary jerking or swaying movements that typically occur at peak dosage and are caused by an overload of dopamine medication. Sometimes dyskinesia can be more troublesome than the Parkinsons symptoms.
Depression And Parkinsonism In An Animal Model Of Neurodegeneration
Early post-natal maternal separation is widely used to create an animal model that exhibits some depressive/anxiety-like behaviors . This established model of depression is useful to study 6-OHDA lesion of the medial forebrain bundle to lesion nigrostriatal DA neurons. We recently investigated the antiparkinsonian effects of Fluvoxamine maleate in a parkinsonian rat model of neurodegeneration associated with anxiety/depressive-like behaviors . Although these studies were a small exploratory open-label trial, they anticipated outcomes on a larger double-blind placebo-controlled study that include non-depressive animals with Parkinsonism. Fluvoxamine maleate treatment has shown potential in decreasing dopaminergic neuronal loss as well as potential to regulate neuronal pro- and anti-inflammation markers in the striatum . Therefore, a combined animal model of chronic stress-induced depression with a 6-OHDA lesioned parkinsonian animal model is an appropriate model to investigate the relationship between depression and PD. This association suggests that the stressor needs to be applied prior to the injection of the neurotoxin 6-OHDA to combine depressive-like behaviors with a potential risk of developing motor-symptoms that characterize Parkinsonism. This combination showed the double advantage of investigating a non-motor symptom as part of an early onset of PD together with the neuroprotective effects of a treatment on the development of the disease.
What Is Parkinson’s Disease
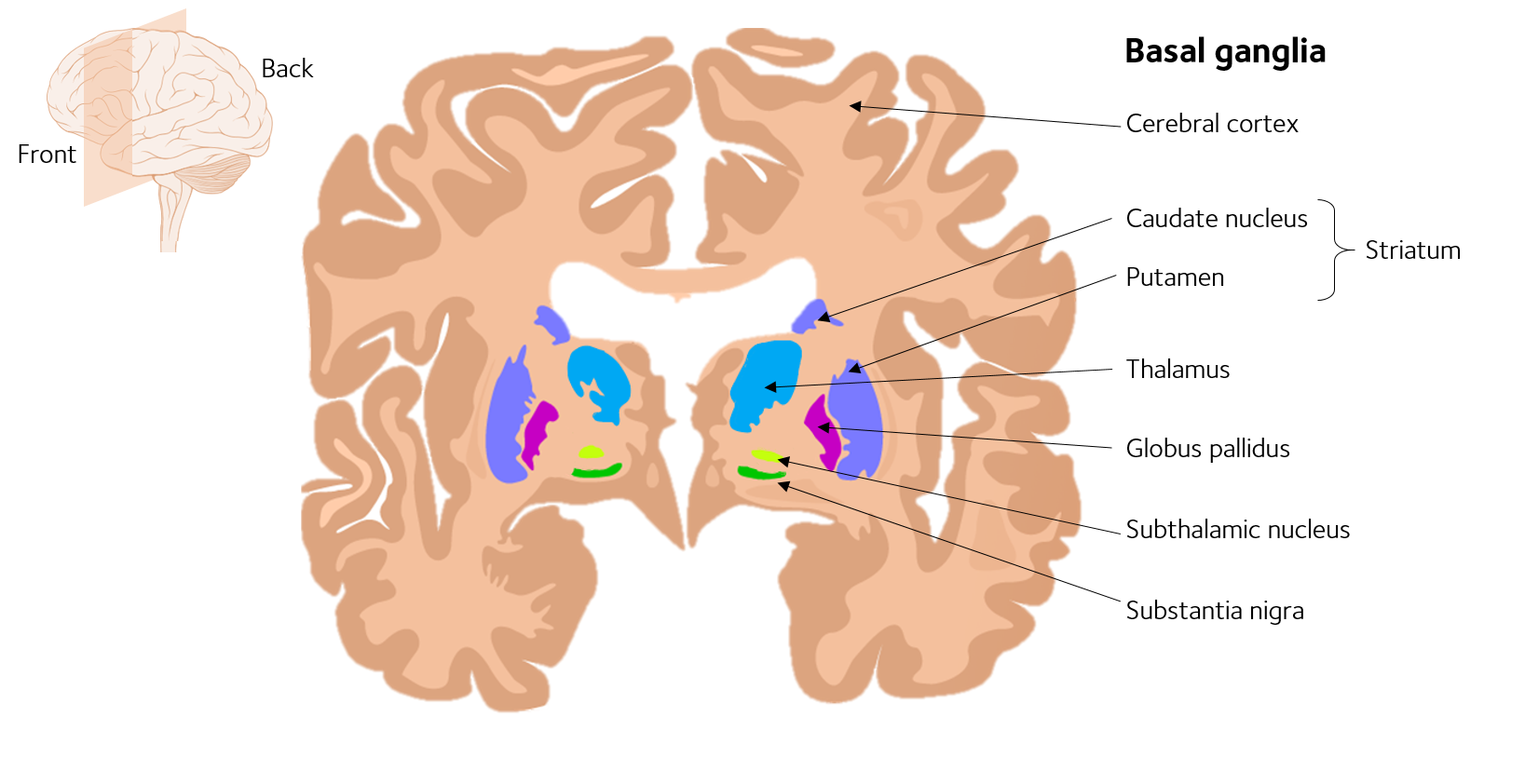
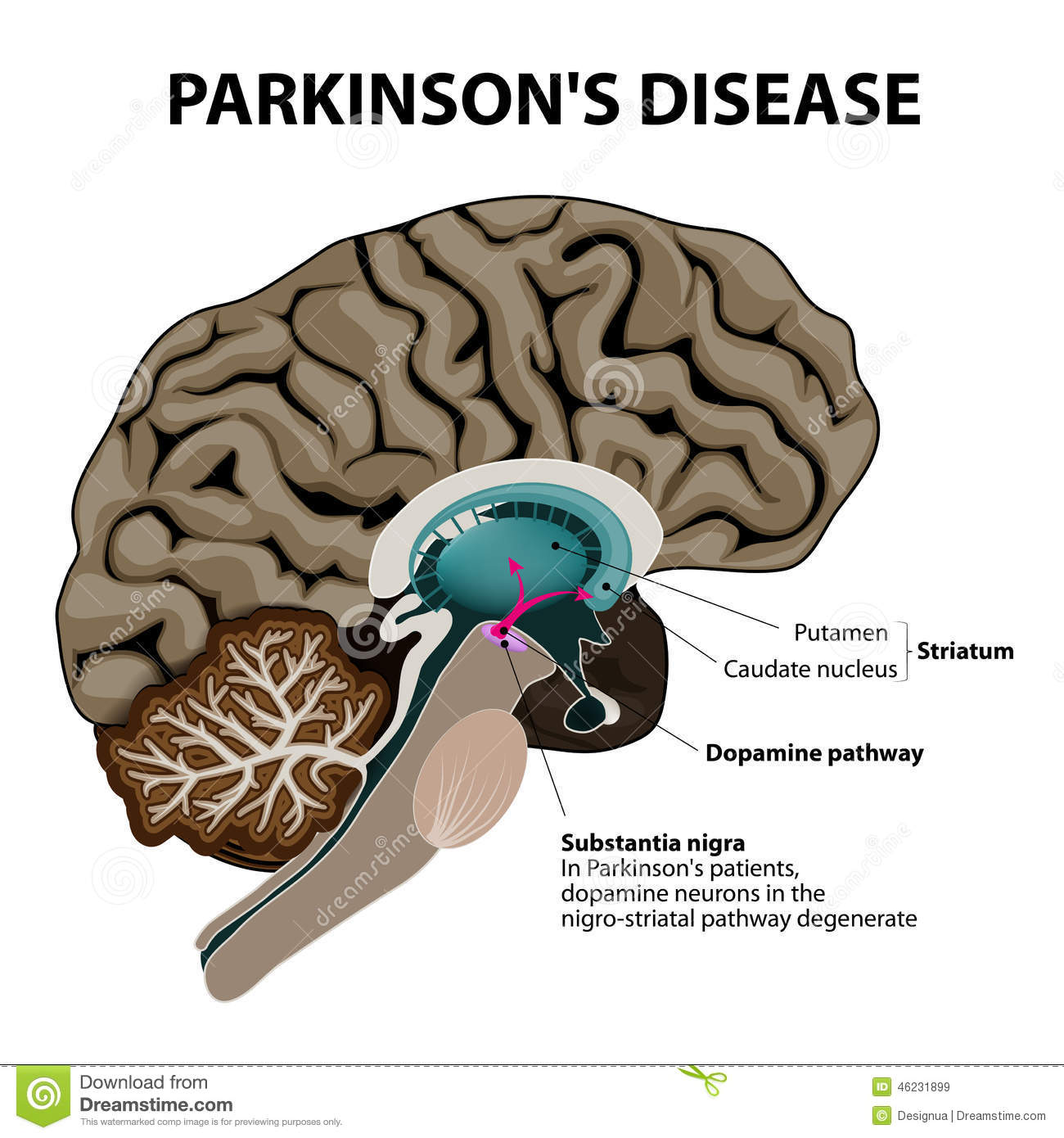
Parkinsons disease is a degenerative, progressive disorder that affects nerve cells in deep parts of the brain called the basal ganglia and the substantia nigra. Nerve cells in the substantia nigra produce the neurotransmitter dopamine and are responsible for relaying messages that plan and control body movement. For reasons not yet understood, the dopamine-producing nerve cells of the substantia nigra begin to die off in some individuals. When 80 percent of dopamine is lost, PD symptoms such as tremor, slowness of movement, stiffness, and balance problems occur.
Body movement is controlled by a complex chain of decisions involving inter-connected groups of nerve cells called ganglia. Information comes to a central area of the brain called the striatum, which works with the substantia nigra to send impulses back and forth from the spinal cord to the brain. The basal ganglia and cerebellum are responsible for ensuring that movement is carried out in a smooth, fluid manner .
The action of dopamine is opposed by another neurotransmitter called acetylcholine. In PD the nerve cells that produce dopamine are dying. The PD symptoms of tremor and stiffness occur when the nerve cells fire and there isn’t enough dopamine to transmit messages. High levels of glutamate, another neurotransmitter, also appear in PD as the body tries to compensate for the lack of dopamine.
Modeling Pd In The Lab
Neurons are difficult to study within the brains of living people, and they cannot be extracted and grown in the lab for research purposes. Based on a Nobel prize-winning scientific breakthrough from 2006, scientists have another way to study neurons from patients. Cells from the skin or blood can now be reprogrammed to an embryonic-like state, called induced pluripotent stem cells, which are then capable of becoming any cell type in the body, including neurons of the brain. For example, blood cells taken from a patient with PD can be reprogrammed into iPS cells in a lab. Those cells can in turn be made into a virtually unlimited supply of dopamine-producing and other types of neurons that can be closely studied by scientists.
Since these cells have the same genetic makeup as the patient, they can exhibit the same disease-processes as the cells in the patient. By comparing the development of iPS cell-derived neurons from PD patients to those from healthy individuals, researchers are studying what goes wrong in PD neurons, and what genes may be involved in the development of the disease. Scientists can use these neurons to further understand disease progression as well as discover new drugs that might be effective at delaying or reversing the disease.
The Nervous System & Dopamine
To understand Parkinson’s, it is helpful to understand how neurons work and how PD affects the brain .
Nerve cells, or neurons, are responsible for sending and receiving nerve impulses or messages between the body and the brain. Try to picture electrical wiring in your home. An electrical circuit is made up of numerous wires connected in such a way that when a light switch is turned on, a light bulb will beam. Similarly, a neuron that is excited will transmit its energy to neurons that are next to it.
Neurons have a cell body with branching arms, called dendrites, which act like antennae and pick up messages. Axons carry messages away from the cell body. Impulses travel from neuron to neuron, from the axon of one cell to the dendrites of another, by crossing over a tiny gap between the two nerve cells called a synapse. Chemical messengers called neurotransmitters allow the electrical impulse to cross the gap.
Neurons talk to each other in the following manner :
What Are The Causes
The cause of Parkinson’s is largely unknown. Scientists are currently investigating the role that genetics, environmental factors, and the natural process of aging have on cell death and PD.
There are also secondary forms of PD that are caused by medications such as haloperidol , reserpine , and metoclopramide .
Dbs And The Role Of Monkey Research
Prior to lesioning a subcortical structure, a surgeon would pass a small amount of electrical current to assess whether or not there was an effect on the symptoms to be treated. This was true for patients with tremor undergoing thalamotomy. One such surgeon, Dr. Alim-Louis Benabid, proposed developing a chronic method to apply stimulation to the target brain region and in conjunction with industry brought DBS to the forefront of surgical therapy for tremor. This evolved to the treatment of PD after initial testing in the MPTP monkey model and has since been considered for a variety of neurological as well as psychiatric disorders. Although DBS was demonstrated effective in alleviating the motor signs associated with PD , little was understood concerning its mechanism of action. With the advent of DBS and its demonstrated benefit to patients with PD, together with the fact it could be performed on both sides of the brain with few side effects compared to lesioning, a search for how it worked, for mechanisms, began. This took on greater importance as the effect of DBS on PD motor signs varied significantly across centers and within centers across patients . To improve clinical outcomes and provide greater consistently in its effect it became critically important to understand how it worked.
Role Of Pet Scans In Diagnosis
Scanning for loss of dopamine, the major chemical responsible for some symptoms of Parkinsons, permits comparison against scans of people who have no dopamine deficiency. By itself, a scan does not make the diagnosis of Parkinson’s but identifies the decline in dopaminergic neurones. It is used by experienced neurologists in cases where the diagnosis of Parkinsons is uncertain.Scans include radionuclide positron emission tomography or by injecting radiopharmaceutical agent in the brain) into a patients veins in a procedure referred to single photon emission computed tomography imaging.
PET scans typically focus on glucose metabolism, and DaT/SPECT scans focus on the activity of the dopamine transporter. Unfortunately, this decline is seen in conditions other than Parkinsons such as Multiple Systems Atrophy and Progressive supranuclear palsy .
Changes In Locus Coeruleus In Parkinson’s Disease
Loss of noradrenergic neuronal and Lewy bodies formation was much higher in locus coeruleus than dopaminergic neuronal loss in PD patients. Resting tremors of PD are due to neuronal loss of LC. About 35% of PD patients were depressed, and loss of noradrenergic pathways underlies the pathophysiology of depression in PD. The pathological changes in LC of PD patients are peculiar and can be differentiated from changes that occur in other neurodegenerative diseases such as schizophrenia. It was reported that simultaneous lesions of dopaminergic system, and LC causes metabolic dysfunction in the cerebral cortex and impairs cognitive functions in PD.
The Neural System Underlying Pd
To illustrate with specific cases the utility of the proposed perspective to better understand proximal causes of PD, in this section we explain how three partially overlapping corticalsubcortical circuits may underlie three important PD symptoms. shows some key components of the BGCtxCer system that are important to study the three symptoms. The schema is not exhaustive of all the possible connections between basal ganglia, cortical, and cerebellar areas. Rather, it focuses on the connections that may have a major role in the three PD symptoms considered here. This is the reason why, for example, the figure indicates SMA/pre-SMA as the only sources of the hyperdirect pathway from cortex to STN, omitting the projections from M1 to STN. The same considerations hold for the , , , which are derived from . The other pathways not considered here might have roles in other aspects of PD symptoms.
When People Talk About Parkinsons They May Mention The Effects It Has On The Substantia Nigra But Did You Know That There Are Other Areas Of The Brain That Are Affected By The Condition
Parkinsons is a condition that causes the gradual loss of the dopamine-producing brain cells of the substantia nigra an area of the brain located just above where the spinal cord meets the midbrain. It is these cells that produce and release the neurotransmitter dopamine, which has a key role in turning thought about movement into action.
While this definition of the condition is useful to briefly explain Parkinsons, the whole story is somewhat more complex. Over the last 30 years, it has become accepted that Parkinsons also causes a number of non-motor symptoms, such as changes in sleep, smell and even the way we think, which likely involve other areas of the brain.
Now scientists are looking at the broader effects of the condition on the brain in an attempt to better understand why people experience different symptoms. The finding could lead us to new treatments that tackle more than just the motor symptoms of the condition.
Functional Magnetic Resonance Imaging
Task-Based fMRI
In addition to changes in the magnitude of brain activity in M1, it has become clearer that PD patients also exhibit disruptions of the pattern of functional connectivity between cortical and subcortical motor regions., The abnormal cortico-subcortical connectivity can be boosted, with recent results demonstrating that an improvement in pedaling rate in PD who had undergone exercise therapy was related to an increase in functional connectivity between M1 and thalamus. A linear increase in motor-related connectivity between the putamen and M1 was also detected following levodopa intake in a cohort of PD who later developed levodopa-induced dyskinesia. This particular case suggests that an increase in functional connectivity is necessary, but if maintained for a long time could lead to dyskinesia. The plasticity of functional connectivity in response temporary motor practice and acute levodopa highlights the need for more long-term interventions targeting the cortex that may help relieve some of the motor signs. Collectively, fMRI and task-fMRI connectivity findings strengthen those obtained with PET by showing that although these measures reflect different characteristics of neural activity, they are feasible for detecting functional abnormalities in M1 that are responsive to intervention.
Resting-State fMRI
Understanding How A Protein Wreaks Havoc In The Brain In Parkinsons Disease
What causes neurons to die in Parkinsons disease?
Parkinsons disease is a long-term neurological condition that affects around 12,000 people in Ireland and between 7 and 10 million people worldwide.
The disease affects the way the brain co-ordinates body movements like walking and talking, but cognitive abilities are also affected.
There is currently no cure for the disease, but researchers at Trinity have recently published findings of a study which may lead to better treatments for this debilitating illness. The paper has been published in the international Cell Press journal Structure.
Neurons in the part of the brain called substantia nigra produce and release a hormone called dopamine. This hormone acts as a messenger between these cells in the substantia nigra and other parts of the brain which control body movements.
If these specialised neurons become damaged or die, the amount of dopamine in the brain is reduced. This means that the parts of the brain that control movement cease to function normally. The only treatment for Parkinsons disease in the last 20 years has been dopamine replacement therapy. This involves providing a substitute to try to increase the levels of the hormone in the brain. However, the treatment is not completely effective and can wear off over time, and it also has side effects,
said Amir Khan, Associate professor, School of Biochemistry and Immunology at Trinity.
Environmental Toxins And Parkinsons Disease
Neuronal cell death in PD may also be triggered by exposure to toxic substances or environmental factors which precipitate the symptoms of the disease as they render the brain vulnerable to subsequent physiological chronic stress . The environmental cause of PD mainly refers to exposure to dopaminergic toxins 6-hydroxydopamine , 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine , paraquat and rotenone as these toxins are known to induce formation of reactive oxygen species and oxidative stress which may result in neuronal cell death .
DA is one of the common neurotransmitters present in most parts of the central nervous system . The mesocortical, mesolimbic, nigrostriatal and tubero-infundibular pathways are the four main pathways that play a key role in dopaminergic signaling . DA cannot cross the blood brain barrier, therefore, it is synthesized from tyrosine which is carried into the brain via amino acid transporters . At the dopaminergic neuron level, tyrosine is then converted into dihydroxyphenylalanine by tyrosine hydroxylase then finally into DA by aromatic L-amino acid decarboxylase . DA is then stored in the vesicle until an action potential allows the vesicle to be discharged into the synapse . Monoamine oxidase is the enzyme that is responsible for breaking down excess DA and is known to similarly act on 6-OHDA inducing oxidative stress resulting in apoptosis .
What Causes Parkinson’s Disease
In the very deep parts of the brain, there is a collection of nerve cells that help control movement, known as the basal ganglia . In a person with Parkinson’s disease, these nerve cells are damaged and do not work as well as they should.
These nerve cells make and use a brain chemical called dopamine to send messages to other parts of the brain to coordinate body movements. When someone has Parkinson’s disease, dopamine levels are low. So, the body doesn’t get the right messages it needs to move normally.
Experts agree that low dopamine levels in the brain cause the symptoms of Parkinson’s disease, but no one really knows why the nerve cells that produce dopamine get damaged and die.
p
The Cerebellum And Non
Many non-motor symptoms, including sensory, autonomic, cognitive and behavioural problems, coexist with the motor signs in Parkinsons disease . Non-motor symptoms exist in up to 60% of patients , and can be primary complaints in Parkinsons disease . Cognitive impairment is common in patients with Parkinsons disease . Hypometabolism in the prefrontal, parietal, temporal and mesolimbic regions was correlated with cognitive impairment in Parkinsons disease . With fluorodeoxyglucose PET and spatial covariance analysis,Huanget al. identified a significant covariance pattern that correlated with cognitive performance, particularly involving executive functioning in Parkinsons disease. This Parkinsons diseaserelated cognitive pattern is characterized by metabolic reductions in frontal and parietal association areas, and increases in the cerebellar vermis and dentate nuclei . Parkinsons diseaserelated cognitive pattern expression increased with worsening of cognitive impairment , but is not correlated with the decline of striatal dopaminergic function . Therefore, the hypermetabolism in the cerebellum might also be a compensatory effort to maintain cognitive function in Parkinsons disease.
Action Sequencing Impairment As Deficit In Timing: Corticalsubcortical Substrates
PD patients have a difficulty in performing action sequences, including completing sequences of heterogeneous movements in correct order., Here we discuss a corticalsubcortical circuit that may underlie this symptom. The circuit, shown on , is mainly based on data supporting the contribution of pre-SMA, SMA, Cer and BG in managing action sequences.,,, Different types of neurons in pre-SMA and SMA help to encode not only where in a sequence the action is but also the conditional links between the previous response and the upcoming response, often in a highly specific manner. In this respect, it has been shown that pre-SMA and SMA neurons respond before some sequences but not others , that some neurons of pre-SMA and SMA respond only to the rank order of a movement in the sequence , and that pre-SMA and SMA cells also encode the number of movements that remain to be made to complete a sequence to obtain a reward.
Importance For Parkinsons Therapy
The researchers identified two areas of the GPe and were able to relate these areas to motor skills and cognitive skills in the mice.
Dr. Byungkook Lim is an associate professor in the Neurobiology Section of the Division of Biological Sciences at UC San Diego and corresponding author of the study.
He explains, our work demonstrates that the distinct neural circuitries in the basal ganglia are differentially involved in the motor and non-motor symptoms of Parkinsonian-like behaviors that occur at different stages of the disease.
This suggests that evaluation of the detailed circuit mechanisms is needed to fully understand the changes in brain during the progression of and could provide better therapeutic strategies for the treatment of .
Dr. Byungkook Lim
The fact that specific neurons could be linked to particular changes in the brain regions of the mice means that it may be possible to develop new treatments for the symptoms of Parkinsons.
In Dr. Lims words, elective manipulation of specific changes can rescue one type of symptom without affecting other symptoms of Parkinsons disease.
How Is Parkinson’s Disease Treated
If a doctor thinks a person has Parkinson’s disease, there’s reason for hope. Medicine can be used to eliminate or improve the symptoms, like the body tremors. And some experts think that a cure may be found soon.
For now, a medicine called levodopa is often given to people who have Parkinson’s disease. Called “L-dopa,” this medicine increases the amount of dopamine in the body and has been shown to improve a person’s ability to walk and move around. Other drugs also help decrease and manage the symptoms by affecting dopamine levels. In some cases, surgery may be needed to treat it. The person would get anesthesia, a special kind of medicine to prevent pain during the operation.
Role Of Serotonin In Parkinsons Disease
Studies have shown that the 5-HT transmission system also undergoes degeneration in PD . The neuronal degeneration in the midbrain raphe nuclei is known to lead to reductions in 5-HT and 5-HT transporter levels in brain areas such as the striatum and prefrontal cortex . However, 5-HT neurons have the ability to store and release DA synthesized from systematically administered DA medication such as levodopa . For instance, in a 6-OHDA lesioned rat model of PD with severe nigrostriatal dopaminergic neuron degeneration, it has been shown that striatal reuptake of levodopa-derived DA can occur through 5-HT transporters . Further, it has been shown that monoamine transporter inhibitors such as selective serotonin reuptake inhibitors can modify striatal dopamine reuptake and metabolism so as to improve motor symptoms of PD . A new treatment approach for PD may therefore consist of blocking 5-HT transporters to enhance and/or prolong the antiparkinsonian effects of drugs that have the potential to increase extracellular DA in the striatum including SSRIs.
Oxidative Stress Neuroinflammation And Parkinsons Disease
Oxidative stress is the result of an imbalance between the production of reactive oxygen species and the body capacity to counteract their harmful effects through neutralization by antioxidant defenses . Brain neurons are constantly exposed to reactive oxygen species and reactive nitrogen species as a result of endogenous or exogenous exposure to oxidative stress . Chronic psychological stress increases neuroinflammation which may facilitate nigral cell death in PD . For instance, under stress conditions, there is evidence that dysfunction of inflammatory markers such as tumor necrosis factor -, interleukin -1, IL-6, IL-10, transforming growth factor – in microglia of patients with depression participates in worsening PD symptoms .
PD research is often directed towards the prevention of DA neuron degeneration . However, all current treatments only address the symptomatic effects of the disease, none of which neither halt nor retard DA neuron degeneration . About 95% of PD cases are sporadic hence caused by environmental factors versus 5% that are inherited . The point of view in favor of exposure to stressful events early in life predisposing an individual to develop neurodegenerative disorders later in life seems to emphasize that PD is much more than just a DA-dependent motor deficit.
Changes In Glial Cells In Parkinson’s Disease
All glial cells can influence the cognitive functions. Structural changes occur in astrocytes in response to physiological and pathological conditions may influence the neurons through nonsynaptic communication with neurons. Altered neuroglial interaction may be the underlying cause for many neurological diseases including PD. Glial response in PD offers both beneficial and hazardous effects.
