Human Ipsc Studies Of Pd Highlight Converging Molecular And Cellular Pathways Across Genetic Subgroups
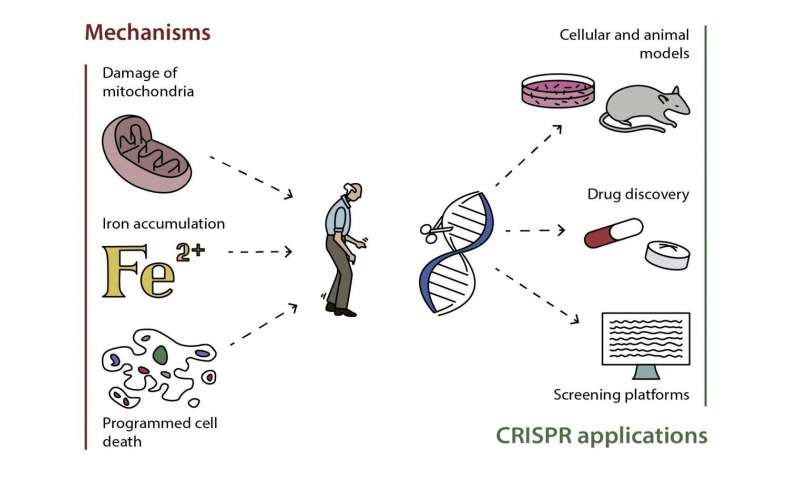
Our analysis of 385 iPSC-derived cell lines from 67 published studies reveals that many PD neuronal phenotypes are shared between genetically heterogeneous familial and sporadic patients . Notably, impairments in mechanisms involved in cellular waste recycling, mitochondrial function, neuronal morphology and physiology, and sensitivity to reactive oxygen species are most common across patient lines with varying genetic predispositions . The studies measured cellular phenotypes that occurred either spontaneously or in response to chemicals mimicking cellular aging and stress . It is important to note that the frequency of reported phenotypes in our meta-analysis may be biased because only few studies reported negative results 31,32,36,37,40,45,48,52,59,64,74,76,86. In addition, most cell lines were not systematically phenotyped without prior hypothesis and thus, there is likely to be an ascertain bias in these phenotypes. Less hypothesis-driven multimodal or omics analysis will help to address such bias41,72,76,77,78,79,80,87,90. Phenotypes caused by genomic predispositions allude to crosstalk and impairments in multiple pathways that act collectively to mediate selective degeneration of dopaminergic neurons in the substantia nigra and will be discussed in detail below.
Fig. 4: Phenotypic insights from iPSC studies of Parkinson’s disease.
Englewood Hospital Will Host Panel Discussion; Jewish Home Family Initiating New Support Group
My father and both of his sisters were afflicted with Parkinson’s disease, a neurological disorder that affects movement by hindering walking and affecting motor control of the hands and head. So the question “Is Parkinson’s a Jewish genetic disorder?” has personal meaning to me.
On December 12, a program addressing that question and other topics related to Parkinson’s disease will take place at Englewood Hospital and Medical Center. Dr. Lana Chahine, a neurologist and Parkinson’s researcher, will speak, and a panel of experts will answer questions on the topic.
Co-sponsored by the Jewish Home Family, the Michael J. Fox Foundation, and Englewood Hospital and Medical Center, the free program is open to physicians, medical and elder care professionals, and members of the community. Parkinson’s patients and their families are particularly encouraged to attend.
The December 12 program also marks the launch of a new community resource, the Center of Excellence in the Care of Parkinson’s. The center has been developed by the Jewish Home Family, a multifaceted eldercare organization serving Bergen, Hudson, and Rockland counties.
“There’s a huge need in the community to deal with this disease,” said Dr. Harvey Gross, a geriatrician who is the medical director of the Jewish Home Family. “We know there are some genes connected with Jewish people, and we felt this program was appropriate to meet the needs of the community, as well as doctors.”
•
The Interplay Between Genomic Predispositions And Environmental Factors Leads To Parkinsons
In the mid-1990s, the connection between PD and underlying genetic mutations was established4,5,150. It is now evident that varying degrees of the interplay between genomic predispositions and aging and cellular stressors impose a risk for disease151 . Previous studies have shown vascular insults to the brain, repeated head trauma, neuroleptic drugs, exposure to pesticides, and manganese toxicity increase the risks of developing symptoms of PD152,153,154. In addition, advancing age can also cause a cascade of stressors within the substantia nigra, which weakens the neurons and their ability to respond to further insults155,156. Ultimately, the uniqueness of the interactions between genes and the environment makes the development of a single treatment for PD difficult as they give rise to a spectrum of neuronal phenotypes that can be unique to individual patients . The development of a model with the ability to replicate the genomic and epigenetic aspects of the disease is crucial . As increasing evidence suggests that genetic mutations are key modulators of disease initiation and progression, the identification and understanding of the various genomic predispositions are required for the development of better-targeted treatments to slow the disease progression.
Fig. 1: A combinatorial spectrum of genetic risks, cellular stressors, and brain cell dysfunctions causes Parkinson’s disease.Full size image
Mitochondrial Recycling In Neurons Is Linked To Genes Mutated In Parkinsons Disease
Introduction
References
1. Genetic Engineering and Biotechnology News. . Genes Mutated in Parkinson’s Disease Linked to Mitochondrial Recycling in Neurons.
2. Nakamura, K . Researchers uncover new mitochondrial recycling pathway that may be linked to Parkinson’s disease. News Medical life Sciences.
3. Li H, Doric Z, Berthet A, Jorgens DM, Nguyen, MK, et al. . Longitudinal tracking of neuronal mitochondria delineates PINK1/ Parkin-dependent mechanisms of mitochondrial recycling and degradation. Science Advances.
4. Ray F Impaired Mitochondrial Recycling Drives Neuron Death in Parkinson’s, Study Indicates. Parkinson’s News Today.
Visit for more related articles at Genetics and Molecular Biology Research
Single Mutation In Recessive Gene Increases Risk Of Earlier Onset Parkinsons Disease
JACKSONVILLE, Fla. — A collaboration of 32 researchers in seven countries, led by scientists at Mayo Clinic’s campus in Florida, has found a genetic mutation they say confers a risk for development of Parkinson’s disease earlier than usual.
The major study, published in Brain, is important because the risk comes from a single mutation in the PTEN-induced putative kinase 1 gene. Investigators had believed that this rare form of Parkinson’s developed only when a person inherited mutations in both PINK1 alleles .
“It took a real international collaboration to solve this puzzle,” he says.
Journalists: Sound bites with Dr. Springer are available in the downloads.
###
People Who Already Have Pd: Should I Get Tested And What Do I Do With The Results
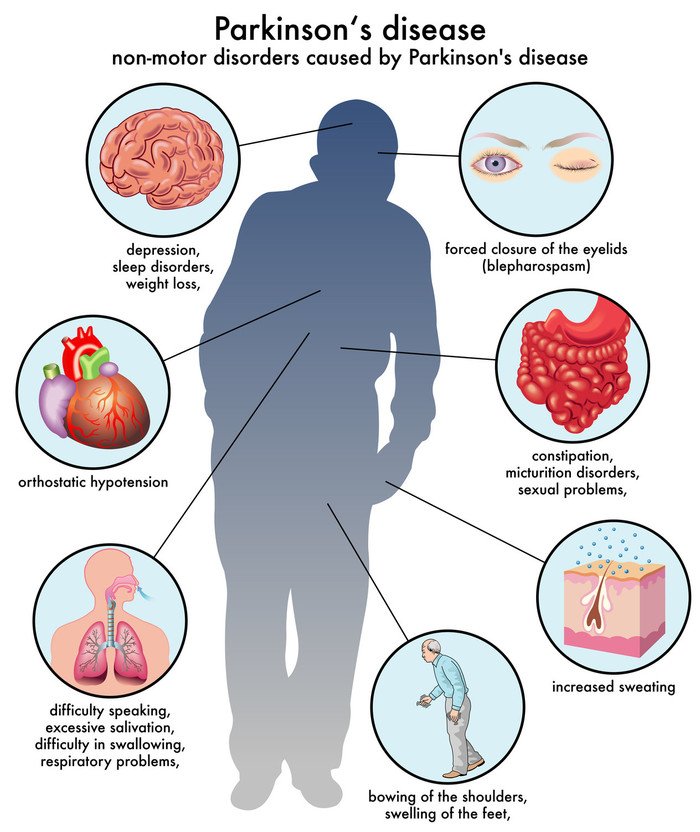
Up until recently, even people with PD with a very extensive family history of PD would not necessarily receive genetic testing because there were no clear uses for the results. There has been research directed at figuring out whether PD caused by or associated with certain mutations have particular clinical characteristics . However, there remains so much variability in clinical characteristics even among people with the same PD mutation, that there are still no clear practical implications in knowing whether a PD patient harbors a particular mutation. There is also, so far, no difference in treatment or management of PD whether or not the patient harbors one of the known mutations. That may change however, with the advent of clinical trials that target particular mutations.
There are two genes that have received particular attention recently because medications are being developed that target those with mutations of these genes.
GBAis a gene that increases the risk of developing PD. The gene encodes for the GBA enzyme, a protein used by the body to break down cellular products. Having two abnormal GBA genes causes Gaucher’s disease, which is characterized by the buildup of these cellular products resulting in fatigue, bone pain, easy bleeding and an enlarged spleen and liver. When a person inherits only one abnormal gene, he or she does not develop Gaucher’s disease, but does incur a small increased risk of PD. Most people with one mutated GBA gene do not develop PD.
Describe How Mature Somatic Cells Can Be Reprogrammed To Become Pluripotent
In modern technology, it is possible to reprogram matured somatic cells in pluripotent cells by introducing factors that can induce cell in situ reprogramming. This technique is highly useful in medicine, including acting as a model for drug and clinical testing, development of drugs.Pluripotent stem cells are the stem cells that can only differentiate into a limited range of differentiated cells. They have the ability to give rise to all somatic cells from ectoderm, mesoderm and endoderm
Parkinson Disease : A Progressive Disorder That Affects The Nervous System
Parkinson disease is known as a progressive disorder that affects the nervous system. Some of the main symptoms of the disorder include tremor, muscular rigidity and slow imprecise movement. On the other hand Alzheimer ‘s disease is the most common cause of dementia. The disorder includes memory loss along with difficulties with thinking, problem-solving or language.Both Alzheimer’s and Parkinson’s have many similarities but evidence may suggest they are separate disorders. Both Parkinson’s and Alzheimer’s
Are Genes Responsible For Monogenic Disorders Also Susceptibility Factors
Associations detected by screening candidate genes in controls and patients cannot always be replicated in follow-up studies, and few candidate genes were confirmed in meta-analysis, because of potential biases and confounding factors, including population stratification, small sample size, misclassification and/or inappropriate statistical methods. Polymorphic variants in SNCA and LRRK2 genes, and heterozygous mutations in the GBA gene, however, have been validated as genetic susceptibility factors .
Nucleotide polymorphisms located close to the promoter region and throughout SNCA have been associated with sporadic PD, although much of the data is equivocal . Rep1 , a mixed nucleotide repeat, 10 kb upstream of the translational start of SNCA , has been confirmed as a risk factor , and synergy between an SNCA variant and a polymorphism in microtubule-associated protein tau , each of which increases the risk for the development of PD, has been detected . The combination of risk genotypes in SNCA and MAPT doubles the risk of PD, further supporting the notion that the related pathways contribute to neurodegenerative diseases . The risk associated with Rep1 does not interact, however, with herbicide exposure, an independent risk factor in PD .
Genetic Predispositions Reducing Differentiation Yield Of Mda Neurons
Will Findings From Pd Ipsc Models Translate To Human Clinical Trials
Pesticide Exposure Increases Risk Of Developing Parkinsons Disease
Link copied
Genetic Predispositions Reducing Differentiation Yield Of Mda Neurons
In vitro neural development was impaired in neural lines derived from patients carrying LRRK2, PRKN, SNCA, and sporadic mutations43,49,74,93. In four independent studies, the differentiation potential of neural progenitor cells derived from patients was significantly reduced, demonstrated by low yields of neurons in comparison with control lines43,49,74,81,94. A recent review presented the idea that PD is attributed to significant neurodevelopmental defects, which may increase the susceptibility for disease onset224. If confirmed, identifying genetic predispositions that contribute to early developmental defects in iPSC-PD may assist the development of novel PD therapies. However, these phenotypes may appear in conflict with other studies53,55,76 capable of generating functional neurons from cell lines with similar mutations. The differences could be due to varying protocols, which may be more or less stressful for the cells.
Will Findings From Pd Ipsc Models Translate To Human Clinical Trials
Given the apprehensions that in vitro studies may be too artificial, human iPSC-derived neural progenitors may be transplanted into animal brains244,245,246,247. Besides ethical barriers, xenografts also raise the possibility that the healthy host tissue compensate for the impairment of the transplanted cells. Yet, if the phenotypes observed in vitro are recapitulated in vivo, pharmacological treatments could be assessed in a systemic environment, with much more realistic dosage and administration methods.
The Genetic Architecture Of Parkinsons Disease In Latino Populations
An international research team led by Cleveland Clinic has presented the most comprehensive characterization of the underlying genetic basis for Parkinson’s disease in Latinos to date, marking an important step towards more inclusive PD genetic research.“Parkinson’s disease impacts all ethnic groups, but since genetic studies have largely been limited to individuals of European and East Asian ancestry, little is known about the genetic architecture of the disease in Latino populations, ” said Ignacio Mata, Ph.D., assistant staff in the Genomic Medicine Institute and lead author on the study. “As we see incidence rates rise in nearly every global region, the importance of greater diversity in Parkinson’s research cannot be overlooked.”In this study, published in Annals of Neurology, Dr. Mata and international collaborators performed the first ever genome-wide association study of Latino PD patients from South America. Their analysis relied on patient data from the world’s largest PD case-control cohort of Latinos, called the Latin American Research Consortium on the Genetics of Parkinson’s Disease , which includes individuals from 35 institutions in 12 countries across Latin America and the Caribbean.
How Environmental Factors And Aging Can Be Recapitulated In Vitro
An obvious limitation of in vitro models is the lack of environmental context. The influence of nongenetic factors is not recapitulated in the basal phenotype of patient-derived neurons. For example, the influence of head trauma of a boxer with sporadic PD will not be recapitulated by default in reprogrammed neurons. An alternative would be to transplant the patient-derived neurons in animals and simulate the trauma on the animal. Similarly, influence of decades of aging of the human brain is difficult to reproduce in vitro in a few months within the boundaries of feasible experimental design. Brains in a dish will always be an imperfect experimental model. However, many tricks can be used to recapitulate the environmental and aging stress in vitro. Table 2 summarizes a list of reagents that have already been used in iPSC neuronal culture to mimic oxidative stress, proteostatic stress, mitochondrial stress, synaptic stress, ER stress, inflammation, and cellular aging. An interesting example is progerin, a truncated form of lamin A associated with premature aging. Increasing the expression of progerin in iPSC neurons can recapitulate at least some aspect of cellular aging in vitro71. Human iPSC-derived dopamine neurons overexpressing progerin displayed specific phenotypes such as neuromelanin accumulation. In addition, PD patient-derived neurons revealed disease-related phenotypes that required both genetic susceptibility and induced-aging in vitro71.
Analysis Of Alzheimer’s Disease: The Neurodegenerative Disease
neurodegenerative disease is the disease in which the neurons become degenerate and causing death of neurons. The Alzheimer’s disease is the first most common neurodegenerative disease. The Alzheimer’s disease is firstly described by Alois Alzheimer in because of this it named Alzheimer’s. The Parkinson’s disease is the second most common neurodegenerative disease National Institute of Environmental Health Sciences . Alzheimer’s disease is the first most common neurodegenerative disease and the
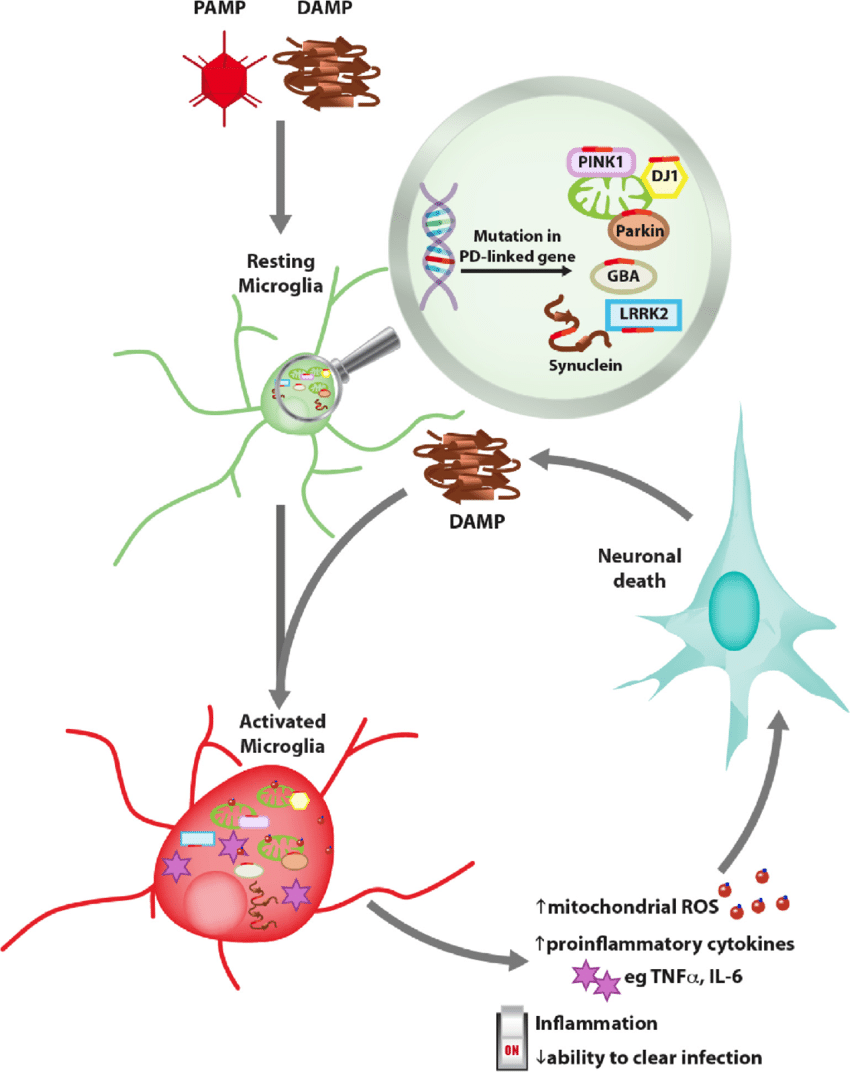
Neuroinflammation Exacerbates Neurodegeneration In Sporadic Pd
Midbrain neurons derived from sporadic patients showed increased susceptibility to the effects of adaptive immune cells72. Sporadic patient neuronal lines co-cultured with T-lymphocytes exhibited substantial signs of cell death mediated by IL-17–IL-17R signaling and activation of NFkB72. Similarly, IL-17 treatment resulted in increased neuronal death72. Inflammation in the central nervous system and periphery are key hallmarks of PD220. Increasing evidence implicates the role of microglia in neuronal loss, though the underlying mechanisms remain to be determined221,222. RNA-seq analysis of astrocytes derived from LRRK2-G2019S iPSCs highlighted dysregulation in genes involved in the extracellular matrix, which may reduce the neuroprotective capacity of astrocytes in PD78. Investigating the role of neuroinflammation in patient-derived microglia may also contribute to the understanding of the selective vulnerability of mDA neurons in sporadic and late-onset PD223.
Review Of Research Paper On Parkinson’s Disease Treatment
down the progression of Parkinson’s disease have largely failed; researchers in this paper maintain this is obviously a direct result of the lack of insight into the pathogenesis of the disease. Parkinson’s disease is the product of the deaths of a number of dopaminergic neurons in the substantia nigra pars compacta region of the brain. But what causes these deaths? In the paper “‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease,” Chen and researchers
Genetic Principles And Exceptions Thereof In Familial Pd
The majority of PD cases are sporadic, i.e., only about 10% of patients report a positive family history . Out of the six genes unequivocally linked to heritable, monogenic PD, mutations in SNCA , and LRRK2 are responsible for autosomal-dominant PD forms, and mutations in Parkin , PINK1 , DJ-1 , and ATP13A2 are accountable for PD that displays an autosomal recessive mode of inheritance.
In general, the inheritance patterns of human disorders are identified by examining the way the disorders are transmitted in the family of the index patient. Such a pedigree analysis requires a careful assembly of the disease records of the family members over several generations, and if possible, examination and sample collection from affected and unaffected individuals from the pedigree. All of the currently known monogenic PD forms are autosomal , which means that they are linked with regions on autosomes .
Pedigree of a PD family that comprises affected members with and without the LRRK2 p.G2019S mutation. Five mutation carriers are unaffected, showing reduced penetrance, two mutation carriers are affected with dystonia, showing variable expressivity, and one affected family member does not have the p.G2019S mutation in LRRK2. Black symbols – affected individuals; white symbols – unaffected individuals; half-filled symbols – individuals with dystonia; + – mutation carriers.
Which Gene Mutations May Be Related To Parkinson Disease
References
Learning From Genetic Analyses Of Pd Casecontrol Studies
We analyzed the reports from 12 international studies94,157,158,159,160,161,162,163,164,165,166,167, totaling 5650 persons living with PD in North America, Europe, and Australia. We confirmed that globally only 15% of patients report a family history of PD symptoms, while the remaining 85% of the PD population are classified as sporadic PD . However, the distinction between genetic predispositions in familial and sporadic PD is blurry. No single-gene mutation in PD has a 100% penetrance. Instead, most likely, multiple genetic risk factors act in synergy to increase the chances of both familial and sporadic PD. Such genetic susceptibilities interplay with aging and environmental factors in both familial and sporadic PD.
Fig. 2: The genomics of Parkinson’s disease: prevalence and penetrance.
a In the world-wide population of people living with PD, ~85% of PD cases are sporadic and the remaining are familial . b Genetic mutations occur at low and varying frequencies in the PD world population . Data represented as the mean±SEM. c GWAS data suggests risk variants in fPD genes tend to be less prevalent in PD cases . d Single nucleotide polymorphisms in over 44 genomic regions show significant association to PD. Each point presents an independent SNP hit associated with PD.
Why Genetic Testing For Parkinsons Disease Is Complex:
- There are many genes that are associated with the development of PD. This list continues to grow as more genes are discovered. Testing of only some of these genes is available in commercial labs.
- The majority of people with PD, even those with a family history of PD, do not harbor one of these identified abnormal genes. The genetic contribution to PD in these people is yet to be discovered.
- For a particular gene there may be a number of different mutations associated with disease, some of which are more common than others. Commercial testing may identify only the most common of the mutations, and therefore not capture everyone who carries a disease-causing mutation.
- Conversely, only particular mutations in a gene may be associated with disease. Commercial testing may identify changes in a gene that may not have clinical consequences. This can be confusing for patients who even after genetic testing may not know whether they harbor a disease-causing mutation.
- Different mutations can be enriched in different ethnic populations. For example, Ashkenazi Jews and North African Berbers have an increased risk of carrying Leucine rich repeat kinase 2 mutations. Glucocerebrosidase mutation frequency also varies greatly with ethnicity and is also increased among Ashkenazi Jews.
In addition to the above, it is important to realize that not all genes associated with PD contribute to disease in the same way:
From Genetics To Molecular Mechanisms Of Parkinsonism
The identification of genes and environmental toxins causative of the parkinsonian syndrome provides a starting point to dissect the molecular mechanisms responsible for pathogenesis. Understanding these mechanisms and the site of action of the pathogenic genes and toxins is critical for designing disease-modifying therapies. We highlight here three mechanisms with a prominent role in PD/parkinsonism: protein dyshomeostasis, mitochondria dysfunction and selective vulnerability.
Genetic Role Not Entirely Known In Affected Families
Genetics very likely plays a role in all types of Parkinson’s disease. However, while having a specific combination of genetics may increase your risk of the disease, it doesn’t necessarily mean that you’ll get it.
Around 15 to 25 percent of people living with Parkinson’s have a family history of the condition, either an immediate or second-degree relation. Having one or more of these relatives will place you at slightly higher risk for Parkinson’s, but it’s still no guarantee you’ll develop the disorder.
Conversely, if you have Parkinson’s, it shouldn’t suggest that any of your kids or grandkids will get the disease either. It merely indicates that their risk is slightly above those without a family history.
In the end, most cases of Parkinson’s don’t have any known cause . While there are forms that seem to run in families, these account for a small percentage of cases — roughly five to 10 percent, all told.
Identification Of New Genes And Risk Factors For Pd
New PD-linked genes or PD risk factors can be identified by gene mapping or candidate gene approaches. Gene mapping in human diseases is the localization of genes underlying the clinical phenotypes of the disease on the basis of correlation with DNA variants , without the need for prior hypotheses about biological function. Genetic mapping methods include linkage analysis and genome-wide association studies. Alternatively, based on their known function, levels of expression, or mode of interaction , some genes can be considered plausible candidates, and as such, tested for in cohorts of patients.
Other Factors Influencing Parkinson’s Disease Risk
Other factors besides genetics can influence someone’s chances of developing Parkinson’s disease, including:
- Age: The risk of developing Parkinson’s disease increases as a person ages.
- Sex: Males have a higher chance of developing Parkinson’s disease than females.
- Family history: First-degree relatives of an individual with Parkinson’s disease have a higher chance of developing Parkinson’s disease.
- Exposure to certain chemicals increases the risk of developing Parkinson’s disease.
Who Should Consider A Genetic Test For Parkinsons
There are two groups of people who might consider getting genetic testing and we will discuss each group separately.
Genetic testing for PD is a common request and a number of commercial labs perform panels of genetic testing for PD. You may ask: “How can I test myself for Parksinon’s?” Whether you’re considering getting a genetic test through your doctor, or performing one at home, it’s important to note that at-home test don’t map the entire gene for mutations. Genetic testing through your doctor will test for GBA, PARK7, SNCA, LRRK2, parkin and PINK1.
Both groups are faced with two questions: Should I get genetic testing? And if so, what should I do with the results? Before we address these two questions, we need to learn more about the complexity of genetic testing in PD.
Epigenomic Alterations Linked With Pd In Patient
Genomic Data Integration And Pag Prioritization
Table 1. Prioritized associated genes in Gene4PD.
Generating Relevant Neuronal Cell Types For Pd
Epigenomic Alterations Linked With Pd In Patient
The ability to capture unique epigenomic alterations associated with PD remains an important challenge. Reprogramming fibroblasts to iPSCs may erase age-associated225 and naive epigenetic signatures which could contribute to sporadic PD pathophysiology226. However, an epigenetic phenotype was reported in iPSC-derived PD patient neurons79,89. Neuronal lines derived from LRRK2 and sporadic patients exhibited epigenomic alterations when compared with healthy controls79. Hypermethylation was prominent in gene regulatory regions associated with the downregulation of transcription factors FOXA1, NR3C1, HNF4A, and FOSL279. Interestingly, LRRK2 mutant and sporadic PD patient neurons shared similar methylation patterns, which were absent in the original donor fibroblasts79. A spontaneous increase in the number of DNA strand breaks and genomic damage89 in PD patient-derived neurons could indirectly impact genomic regulation.
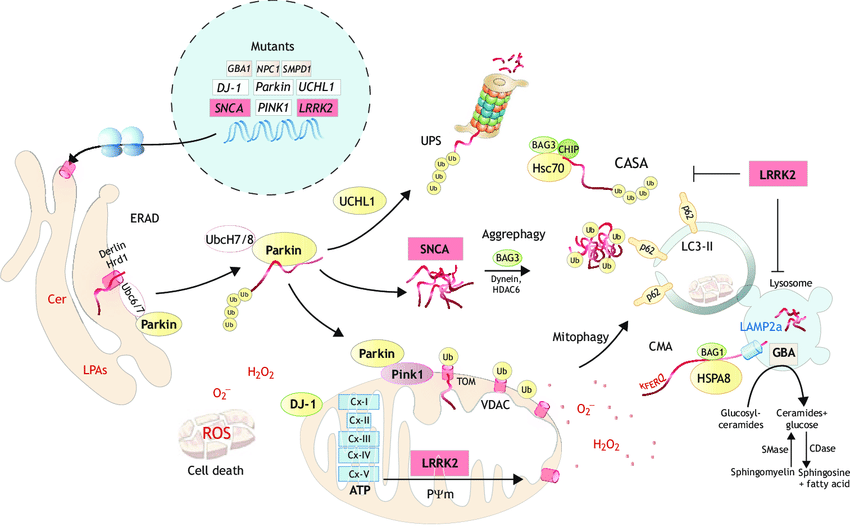
Autosomal Recessive Genes In Parkinsons Disease
- Parkin – Parkin is known as an ubiquitin ligase. It adds ubiquitin molecules to proteins that tag damaged proteins for degradation in the cell so the proteins get chopped up into their more basic form. More than 200 mutations in Parkin have been identified that can lead to PD. Some of the mutations cause a juvenile form of PD , while other mutations cause a later onset of the disease.2
- PINK1 – The PINK1 gene codes for a protein called PTEN induced putative kinase 1. More than 70 mutations in the PINK1 gene can cause PD, and mutations on PINK1 are associated with an early onset of the disease . It is thought that both PINK1 and Parkin act together to target damaged mitochondria for destruction. Mitochondria are responsible for producing energy in the cell in the form of ATP. When they are damaged, they can produce what are called reactive oxygen species which can be toxic for proteins. Mutations in PINK1 and Parkin may prevent the cell from getting rid of damaged mitochondria, causing damage and possibly death of neurons.2
- ATP13A2 – Recessive mutations on the ATP13A2 gene are associated with Kufor-Rakeb syndrome, a rare form of juvenile parkinsonism.2
Generating Relevant Neuronal Cell Types For Pd
The cellular reprogramming toolbox for researchers is rapidly expanding and includes a panoply of neuronal differentiation protocols to generate cells representing various brain regions21. PD is a debilitating motor system disorder resulting from the selective degeneration of midbrain dopamine neurons located in the substantia nigra pars compacta. Protocols have been established to specifically generate dopaminergic neurons and brain cells with a midbrain molecular profile195,196.
Key Gene Mutations Associated With Parkinson’s
There are forms of Parkinson’s that appear to be influenced by genetic defects that run in families. We tend to see this with early-onset forms of the disease wherein symptoms being to appear far earlier than average onset age of 60.
One type of genetic mutation associated with familial parkinsonism is in the so-called SNCA gene. This is the gene linked with the production of alpha-synuclein protein, a biomolecule which can contribute to abnormalities in nerve cells. While rare in the general population, the SNCA gene mutation has been identified in around two percent of families affected by Parkinson’s.
In 2004, scientists discovered a similar genetic mutation in a number of families in whom multiple members had been affected. The so-called LRRK2 mutation is today linked to about one to two percent of all Parkinson cases, mostly affecting people of Jewish, Ashkenazi, North African Arab-Berber, or Basque origin.
Another mutation involving the GBA gene is already known to cause Gaucher’s disease . Research has since shown that the GBA mutation is present in a significant number of people with Parkinson’s, suggesting a causal link between the mutation and the disease.
Can Parkinsons Be Passed From Parent To Child
It’s rare for Parkinson’s disease to be passed down from parent to child. Most cases of Parkinson’s aren’t hereditary. But people who get early-onset Parkinson’s disease are more likely to have inherited it.
Having a family history of Parkinson’s disease may increase the risk that you’ll get it. This means that having a parent or sibling with Parkinson’s slightly increases the risk.
In most cases, the cause of Parkinson’s disease remains unknown. But researchers have identified multiple risk factors that can increase your chances of getting this disease.
Risk factors for Parkinson’s disease include:
- mutations in specific genes associated with Parkinson’s
- having a family history of Parkinson’s or a first-degree family member with Parkinson’s
- being older, especially above the age of 60
- exposure to herbicides and pesticides
- being assigned male at birth
- history of brain injury
What Genes Are Linked To Parkinson’s Disease
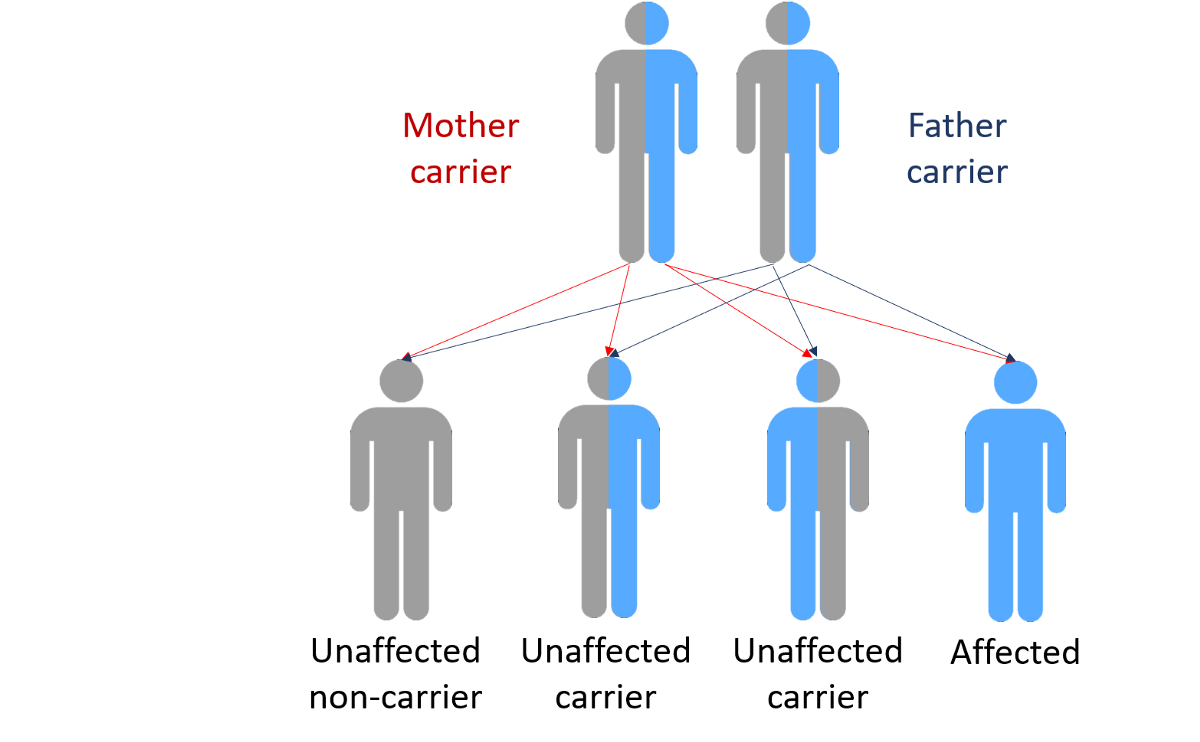
In 1997, we studied a large family that came from a small town in Southern Italy in which PD was inherited from parent to child . We found the gene that caused their inherited Parkinson’s Disease and it coded for a protein called alpha-synuclein. If one studies the brains of people with PD after they die, one can see tiny little accumulations of protein called Lewy Bodies . Research has shown that there is a large amount of alpha-synuclein protein in the Lewy Bodies of people who have non-inherited PD as well as in the brains of people who have inherited PD. This immediately told us that alpha-synuclein played an important role in all forms of PD and we are still doing a lot of research to better understand this role.
Currently, seven genes that cause some form of Parkinson’s disease have been identified. Mutations in three known genes called SNCA , UCHL1 , and LRRK2 and another mapped gene have been reported in families with dominant inheritance. Mutations in three known genes, PARK2, PARK7 , and PINK1 have been found in affected individuals who had siblings with the condition but whose parents did not have Parkinson’s disease . There is some research to suggest that these genes are also involved in early-onset Parkinson’s disease or in dominantly inherited Parkinson’s disease but it is too early yet to be certain.
What Determines Who Gets Parkinson’s Disease
In most cases inheriting a non-working copy of a single gene will not cause someone to develop Parkinson’s disease. We believe that many other complicating factors such as additional genes and environmental factors determine who will get the condition, when they get it and how it affects them. In the families we have studied, some people who inherit the gene develop the condition and others live their entire lives without showing any symptoms. There is a lot of research on genes and the environment that is attempting to understand how all these factors interact.
Genetic Testing in Parkinson’s Disease
Genetic testing has recently become available for the parkin and PINK1 genes. Parkin is a large gene and testing is difficult. At the current stage of understanding, testing is likely to give a meaningful result only for people who develop the condition before the age of 30 years. PINK1 appears to be a rare cause of inherited Parkinson’s disease. A small percentage of those developing the condition at an early age appear to carry mutations in the PINK1 gene. Genetic testing for the PARK7, SNCA and LRRK2 genes is also available.
Additional Resources
