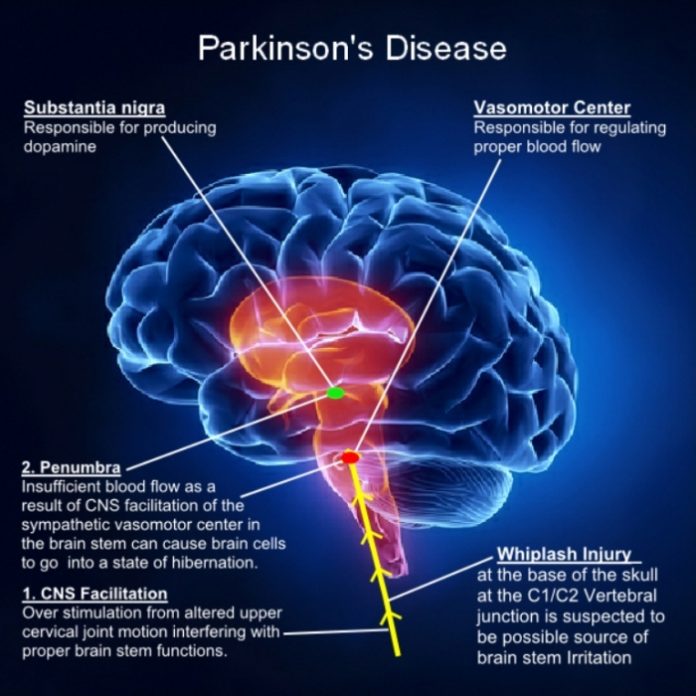
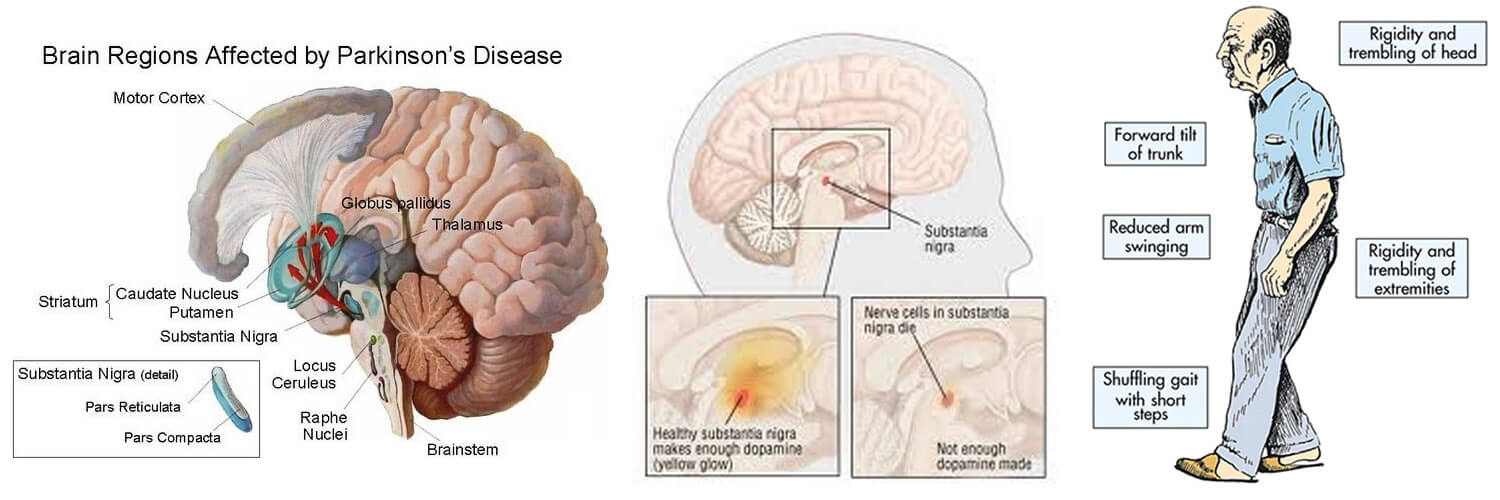
When People Talk About Parkinsons They May Mention The Effects It Has On The Substantia Nigra But Did You Know That There Are Other Areas Of The Brain That Are Affected By The Condition
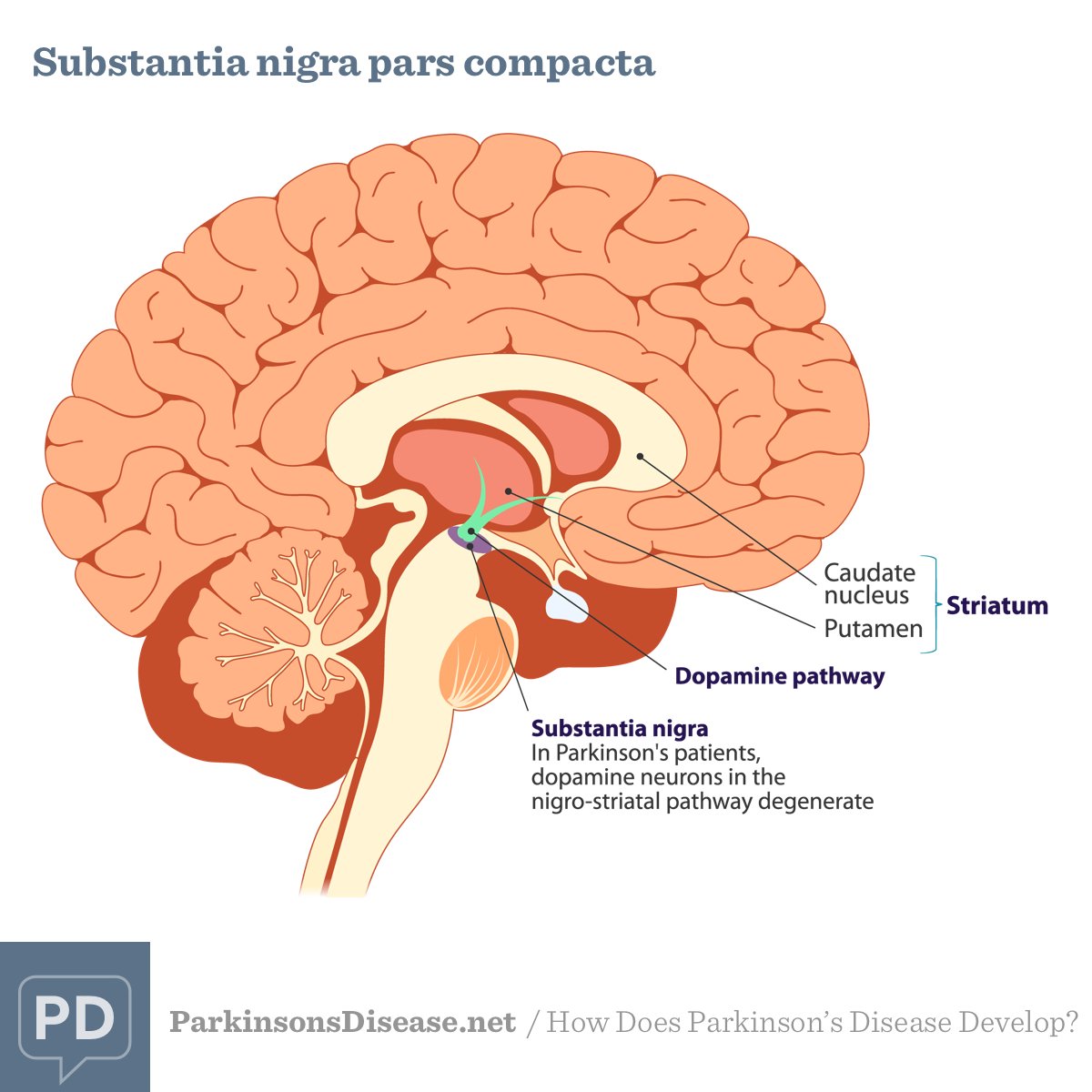
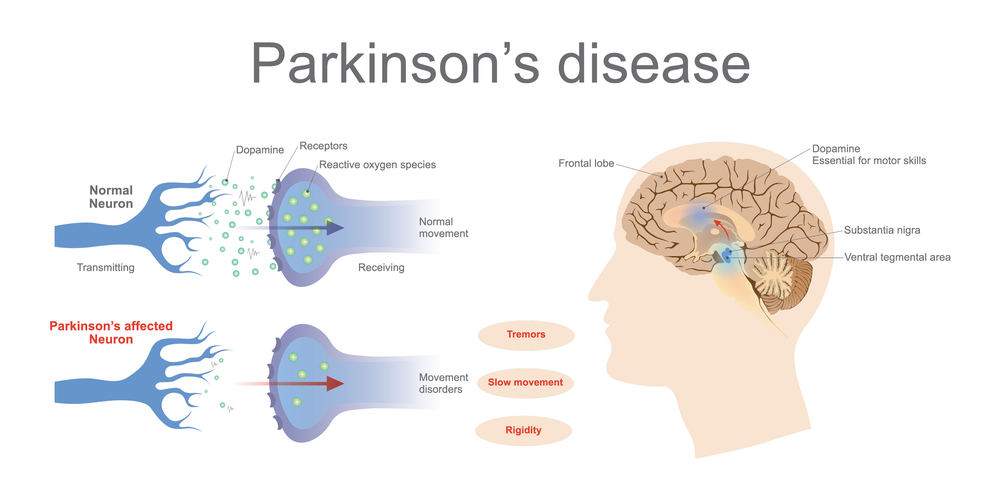
Parkinson’s is a condition that causes the gradual loss of the dopamine-producing brain cells of the substantia nigra — an area of the brain located just above where the spinal cord meets the midbrain. It is these cells that produce and release the neurotransmitter dopamine, which has a key role in turning thought about movement into action.
While this definition of the condition is useful to briefly explain Parkinson’s, the whole story is somewhat more complex. Over the last 30 years, it has become accepted that Parkinson’s also causes a number of non-motor symptoms, such as changes in sleep, smell and even the way we think, which likely involve other areas of the brain.
Now scientists are looking at the broader effects of the condition on the brain in an attempt to better understand why people experience different symptoms. The finding could lead us to new treatments that tackle more than just the motor symptoms of the condition.
Brain Regional Specific Metabolic Disturbance Was Involved In Parkinsons Disease
To further explore discriminating features and potential biomarkers in brain function and disease, the supervised PLS-DA analyses were applied for six different brain regions from three groups . Moreover, a mixed-model analysis was applied to evaluate the effects of dopamine loss and L-dopa replacement on metabolite alterations in the PD and LID rats and the detailed results were shown in Figure 5 and listed in Supplementary Tables S1–S6. As predicted, a clear separation between control and PD rats was observed in the midbrain and striatum . Accordingly, using hierarchical clustering on the profile of the identified 21 metabolites, the metabolic profile of midbrain and striatum was strictly separated between control and PD rats, several interesting metabolite clusters became apparent as well . One of these clusters contained glutamate and glutamine, and this alteration in the glutamate metabolism pathway reflected neurotransmitter homeostasis in the brain . In addition, various products of energy metabolism pathways in the striatum were altered in PD rats .
Figure 4. PLS-DA score plots of Con, PD and LID groups in the different brain regions: Mid, midbrain; Rstr, right striatum; Rcor, right cortex; Rhip, right hippocampus; Rcer, right cerebellum; Hyp, hypothalamus.
Research Is Underway To Further Understand The Cardiac Effects Of Parkinsons
It is possible to image the sympathetic nervous system of the human heart by injecting a radioactive tracer, meta-iodo-benzyl-guanidine, . Development of this technique, known as MIBG cardiac imaging, holds much promise as a test to confirm the diagnosis of PD , to identify those who are at risk of developing PD in the future, and to distinguish PD from related disorders. MIBG cardiac imaging is still considered an experimental procedure for detection of PD and is not yet in use as a clinical tool for this purpose.
A recent research study was conducted in monkeys in which the destruction of the sympathetic nerves of the heart was chemically induced to mimic the changes that are seen in PD. The cardiac system was then imaged using a number of new-generation radioactive tracers, which bind to markers of inflammation and oxidative stress. This model system may help to shed light on the molecular changes that accompany the loss of the sympathetic nerves of the heart and can also be used to track the response of the cardiac system to therapeutic agents.
How Parkinsons Disease Affects The Autonomic Nervous System And The Heart
In PD, there are two major reasons why the automatic control of the cardiac system is impaired. First, areas of the brain that control this system often contain Lewy bodies and have undergone neurodegeneration. In addition, the autonomic nervous system itself is directly affected by Lewy body-like accumulations and neurodegeneration. This means, when the baroreceptors in the heart and carotid artery sense a drop in blood pressure and try to generate a signal to the heart and blood vessels to increase the blood pressure, the message may not get through. This results in neurogenic orthostatic hypotension , or drops in blood pressure upon standing due to autonomic nervous system dysfunction. There are no medications that can cure nOH by restoring the autonomic nervous system in PD. nOH however, can be treated. Read more about nOH and its treatments here.
Structural problems of the heart such as coronary artery disease or cardiomyopathy are not thought to be part of the pathology of PD, although of course, could co-exist with PD.
The Heart Of The Matter: Cardiovascular Effects Of Parkinsons Disease
It has long been understood that Parkinson’s disease does not just cause movement symptoms, but also causes a litany of non-motor symptoms with effects throughout the body. One of the organ systems that is affected is the cardiac system, encompassing the heart, as well as the major and minor blood vessels. I received this topic as a suggestion from a blog reader and we will be discussing this important issue today. Please feel free to .
Parkinsons Diseaseassociated Pathological Changes In The Cerebellum
The presence of dopaminergic innervation and dopamine D1–3 receptors in the cerebellum has been proven . The cerebellum receives a dopaminergic projection from the ventral tegmental area/substantia nigra pars compacta . Pathological changes in the cerebellum following dopaminergic degeneration were reported in patients with Parkinson’s disease and animal models.Rollandet al. showed that degeneration of nigrostriatal dopaminergic neurons causes dysfunction of both the basal ganglia–thalamic and cerebello-thalamic pathways in 6-hydroxydopamine-lesioned rats and MPTP monkeys. Neuronal degeneration in the cerebellum was shown in an MPTP mouse model , characterized by the loss of Nissl-stained Purkinje cells and aggravated by the number of repeated MPTP injections. An MPTP insult also induced the loss of calcium-binding positive Purkinje cells in monkeys . A recent study found that persistent hyperactivation of Purkinje cells correlated with the level of dopaminergic neuronal loss in the substantia nigra in chronic parkinsonian monkeys .
Data Processing Of Nmr Spectra And Multivariate Pattern Recognition
NMR spectra were reduced to integrated regions with a width of 0.01 ppm corresponding to the region of ? 10–0.5. The regions of 4.7–5.2 ppm was removed to eliminate artifacts related to the residual water resonance. The remaining spectral segments were then normalized to the total sum of the spectral intensity to partially compensate for differences in the concentration of many metabolites in the samples. Before multivariate data analysis, the integral values were mean-centered and Pareto-scaled . NMR data sets were imported into SIMCA-P + 12.0 software for multivariate statistical analysis, including principal component analysis discriminant analysis and partial least squares-discriminant analysis . And another data reduced to integrated regions with a width of 0.0015 ppm width corresponding to the region of ? 10–0.5 were used for quantitative analysis.
Synaptic Plasticity Is Impaired In Parkinsons Disease And Lid
It was noticed that there was a disorder of neurotransmitters in the PD and LID rats, we wondered if the synaptic plasticity, which controls the release and recycle of neurotransmitters was also involved in the PD and LID disease. SYP, a standard presynaptic marker, which was responsible for synapse formation and neurotransmitter recycle . Immunofluorescence staining was performed to visually evaluate the expression of SYP in the right cortex of PD and LID rats. The immunofluorescence staining results showed that the density of SYP in the right cortex of PD and LID rats was lower than that in the Con rats . Accordingly, the results of real-time RT-PCR also showed that the gene expression of SYP in the right cortex of PD and LID rats was significantly decreased compared to Con rats .
Figure 7. Gene and protein expression of synaptophysin in the right cortex of control, PD and LID rats. Immunofluorescence staining of SYP in the cortex : labeling for SYP and staining nuclei with DAPI . Magnification: X400. Scale bar: 100 ?m. Real-time RT-PCR results showed that the expression of SYP in the right cortex in the experimental groups were significantly decreased compared with that in the control group. Differences among the three groups were analyzed using univariate ANOVA. *P< 0.05 is compared with control group.
What Lifestyle Changes Can I Make To Ease Parkinsons Symptoms
Exercise: Exercise helps improve muscle strength, balance, coordination, flexibility, and tremor. It is also strongly believed to improve memory, thinking and reduce the risk of falls and decrease anxiety and depression. One study in persons with Parkinson’s disease showed that 2.5 hours of exercise per week resulted in improved ability to move and a slower decline in quality of life compared to those who didn’t exercise or didn’t start until later in the course of their disease. Some exercises to consider include strengthening or resistance training, stretching exercises or aerobics . All types of exercise are helpful.
Eat a healthy, balanced diet: This is not only good for your general health but can ease some of the non-movement related symptoms of Parkinson’s, such as constipation. Eating foods high in fiber in particular can relieve constipation. The Mediterranean diet is one example of a healthy diet.
Preventing falls and maintaining balance: Falls are a frequent complication of Parkinson’s. While you can do many things to reduce your risk of falling, the two most important are: 1) to work with your doctor to ensure that your treatments — whether medicines or deep brain stimulation — are optimal; and 2) to consult with a physical therapist who can assess your walking and balance. The physical therapist is the expert when it comes to recommending assistive devices or exercise to improve safety and preventing falls.
Improve the quality of your sleep.
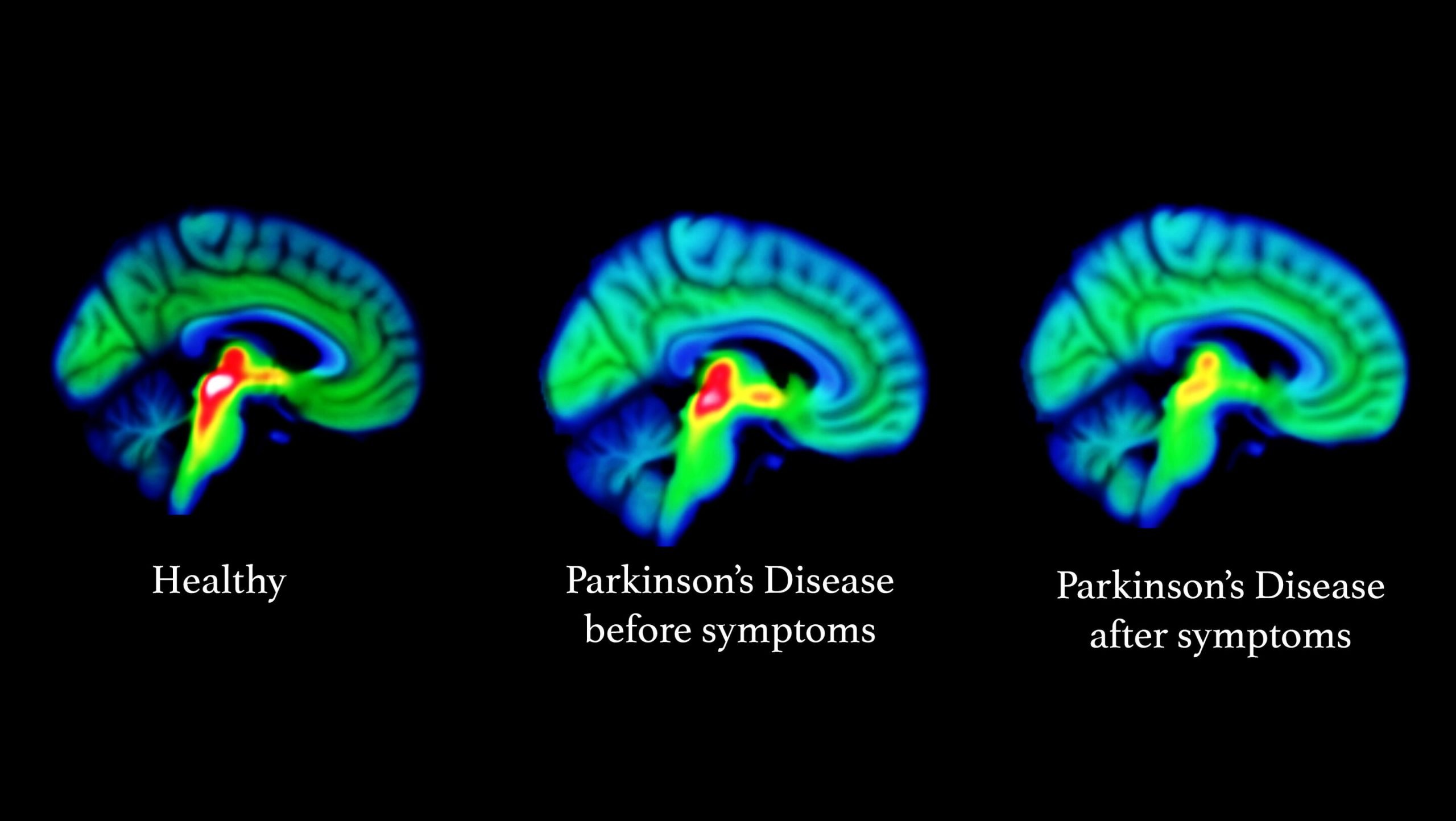
Parkinson’s Disease Brain Vs Normal Brain: What’s Different
It’s not yet possible to spot the difference between a brain with Parkinson’s and a normal, “healthy” brain on an MRI scan. However, since Lewy bodies were first found in the substantia nigra in 1927, doctors have known they are a feature of Parkinson’s disease. The presence of these Lewy bodies is thought to be what separates people with Parkinson’s disease from the general population. However, Lewy bodies can only be diagnosed with certainty during a brain autopsy after death.
The Cerebellum As A Target For Parkinsons Disease Treatment
While cerebellar dysfunction might contribute to some motor and non-motor signs in Parkinson’s disease, a possible approach for treating parkinsonian symptoms is to attempt to normalize cerebellar function. Surgical treatment, such as deep brain stimulation of the subthalamic nucleus or globus pallidus improves the motor signs and normalizes cerebellar activation. Levodopa administration can also normalize the activity and connectivity in the cerebello-thalamo-cortical circuit . However, whether it is reduced compensation or alleviation of pathological impairment as a consequence of effective treatment remains unclear. Suppressing cerebellar activity should theoretically answer the question: improvement would mean that the cerebellum is contributing to the manifestations; worsening would mean that the cerebellar activity is compensatory. We suppose that if the main efforts of the cerebellum in Parkinson’s disease are compensatory, suppression of cerebellar activity should be accompanied by further impairments of Parkinson’s disease symptoms.
Understanding The Neurologic Control Of The Cardiac System
Before we explore this issue, let’s first learn a bit about the autonomic nervous system and about the cardiac system’s place within it. The ANS is part of the peripheral nervous system, a network of nerves throughout the body. The ANS exerts control over functions that are not under conscious direction such as respiration, heart function, blood pressure, digestion, urination, sexual function, pupillary response, and much more. The ANS is further subdivided into the parasympathetic nervous system and the sympathetic nervous system. Both the parasympathetic and sympathetic nervous systems regulate most major organs. Often, they have opposite effects, with the sympathetic nervous system activating a system and the parasympathetic system calming it down.
One of the systems controlled by the ANS is cardiac regulation. Blood pressure sensors, known as baroreceptors, reside in the heart as well as in the carotid artery, the major artery in the neck. If the baroreceptors sense a change in the blood pressure, a signal is sent to particular areas in the brain. From there, the autonomic nervous system sends signals to the heart to control heart rate and cardiac output. Signals are also sent to the blood vessels to change the size of their diameter, thereby regulating blood pressure.
What Are The Surgical Treatments For Parkinsons Disease
Most patients with Parkinson’s disease can maintain a good quality of life with medications. However, as the disease worsens, medications may no longer be effective in some patients. In these patients, the effectiveness of medications becomes unpredictable – reducing symptoms during “on” periods and no longer controlling symptoms during “off” periods, which usually occur when the medication is wearing off and just before the next dose is to be taken. Sometimes these variations can be managed with changes in medications. However, sometimes they can’t. Based on the type and severity of your symptoms, the failure of adjustments in your medications, the decline in your quality of life and your overall health, your doctor may discuss some of the available surgical options.
What Is The Outlook For Persons With Parkinsons Disease
Although there is no cure or absolute evidence of ways to prevent Parkinson’s disease, scientists are working hard to learn more about the disease and find innovative ways to better manage it, prevent it from progressing and ultimately curing it.
Currently, you and your healthcare team’s efforts are focused on medical management of your symptoms along with general health and lifestyle improvement recommendations . By identifying individual symptoms and adjusting the course of action based on changes in symptoms, most people with Parkinson’s disease can live fulfilling lives.
The future is hopeful. Some of the research underway includes:
- Using stem cells to produce new neurons, which would produce dopamine.
- Producing a dopamine-producing enzyme that is delivered to a gene in the brain that controls movement.
- Using a naturally occurring human protein – glial cell-line derived neurotrophic factor, GDNF – to protect dopamine-releasing nerve cells.
Many other investigations are underway too. Much has been learned, much progress has been made and additional discoveries are likely to come.
Which Medicines Are Used To Treat Parkinson’s Disease
Guidelines released by the Scottish Intercollegiate Guidelines Network recommend starting with a dopamine agonist, levodopa with a dopa-decarboxylase inhibitor or a monoamine-oxidase inhibitor. Other medicines are also sometimes used, usually in addition to one of these three main types of medication.
What Medications Are Used To Treat Parkinsons Disease
Medications are the main treatment method for patients with Parkinson’s disease. Your doctor will work closely with you to develop a treatment plan best suited for you based on the severity of your disease at the time of diagnosis, side effects of the drug class and success or failure of symptom control of the medications you try.
Medications combat Parkinson’s disease by:
- Helping nerve cells in the brain make dopamine.
- Mimicking the effects of dopamine in the brain.
- Blocking an enzyme that breaks down dopamine in the brain.
- Reducing some specific symptoms of Parkinson’s disease.
Levodopa: Levodopa is a main treatment for the slowness of movement, tremor, and stiffness symptoms of Parkinson’s disease. Nerve cells use levodopa to make dopamine, which replenishes the low amount found in the brain of persons with Parkinson’s disease. Levodopa is usually taken with carbidopa to allow more levodopa to reach the brain and to prevent or reduce the nausea and vomiting, low blood pressure and other side effects of levodopa. Sinemet® is available in an immediate release formula and a long-acting, controlled release formula. Rytary® is a newer version of levodopa/carbidopa that is a longer-acting capsule. The newest addition is Inbrija®, which is inhaled levodopa. It is used by people already taking regular carbidopa/levodopa for when they have off episodes .
Drugs And Medication Used To Treat Parkinsons Disease
A number of different drugs can be used to treat Parkinson’s.
Levodopa
Levodopa is the most common treatment for Parkinson’s. It helps to replenish dopamine.
About 75 percent of cases respond to levodopa, but not all symptoms are improved. Levodopa is generally given with carbidopa.
Carbidopa delays the breakdown of levodopa which in turn increases the availability of levodopa at the blood-brain barrier.
Dopamine agonists
Dopamine agonists can imitate the action of dopamine in the brain. They’re less effective than levodopa, but they can be useful as bridge medications when levodopa is less effective.
Drugs in this class include bromocriptine, pramipexole, and ropinirole.
Anticholinergics
Anticholinergics are used to block the parasympathetic nervous system. They can help with rigidity.
Benztropine and trihexyphenidyl are anticholinergics used to treat Parkinson’s.
Amantadine
Amantadine can be used along with carbidopa-levodopa. It’s a glutamate-blocking drug . It offers short-term relief for the involuntary movements that can be a side effect of levodopa.
COMT inhibitors
Catechol O-methyltransferase inhibitors prolong the effect of levodopa. Entacapone and tolcapone are examples of COMT inhibitors.
Tolcapone can cause liver damage. It’s usually saved for people who do not respond to other therapies.
Ectacapone does not cause liver damage.
Stalevo is a drug that combines ectacapone and carbidopa-levodopa in one pill.
MAO-B inhibitors
Behavior Tests In Experimental Models Of Pd And Lid
To mimic dyskinesia of PD patients, male SD rats were unilaterally injected with 6-OHDA in the right MFB, causing a nearly complete depletion of ipsilateral dopamine, while the rats in Con group were got same Sham-operation but injected with vehicle . Three weeks later, motor symptoms were validated using apomorphine-induced rotation test . Then the LID rats were intraperitoneally administrated L-dopa with benserazide once a day for 3 weeks and observed progressive AIMs. Forelimb function test was conducted at days 3, 8, 13, 18 after L-dopa/benserazide treatment, results showed that the percent of left forelimb use of total wall contacts of rats in Con group is around 50%, while the percent of left forelimb use was significantly reduced in PD rats with a partial lesion of the nigrostriatal pathway. After the treatment of L-dopa, the percent of left forelimb use of rats in the LID group was significantly increased compared to that in the PD group . These results suggested that PD rats showed reduced forelimb motor function, and L-dopa treatment is quite effective in alleviating PD behavioral symptoms.
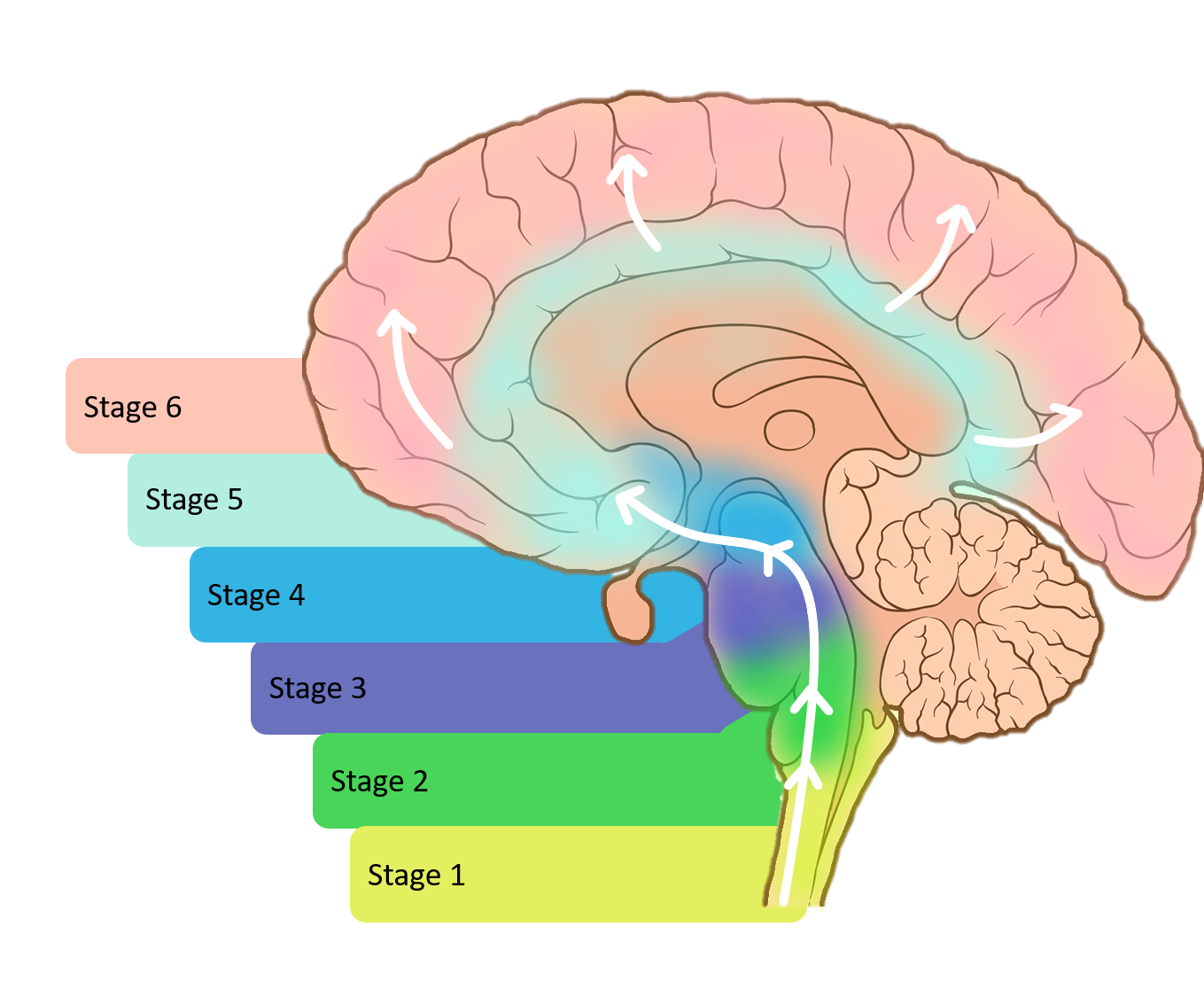
What Are The Different Stages Of Parkinsons Disease
Each person with Parkinson’s disease experiences symptoms in in their own unique way. Not everyone experiences all symptoms of Parkinson’s disease. You may not experience symptoms in the same order as others. Some people may have mild symptoms; others may have intense symptoms. How quickly symptoms worsen also varies from individual to individual and is difficult to impossible to predict at the outset.
In general, the disease progresses from early stage to mid-stage to mid-late-stage to advanced stage. This is what typically occurs during each of these stages:
Early stage
Early symptoms of Parkinson’s disease are usually mild and typically occur slowly and do not interfere with daily activities. Sometimes early symptoms are not easy to detect or you may think early symptoms are simply normal signs of aging. You may have fatigue or a general sense of uneasiness. You may feel a slight tremor or have difficulty standing.
Often, a family member or friend notices some of the subtle signs before you do. They may notice things like body stiffness or lack of normal movement slow or small handwriting, lack of expression in your face, or difficulty getting out of a chair.
Mid stage
Mid-late stage
Standing and walking are becoming more difficult and may require assistance with a walker. You may need full time help to continue to live at home.
Advanced stage
Changes In Locus Coeruleus In Parkinson’s Disease
Loss of noradrenergic neuronal and Lewy bodies formation was much higher in locus coeruleus than dopaminergic neuronal loss in PD patients. Resting tremors of PD are due to neuronal loss of LC. About 35% of PD patients were depressed, and loss of noradrenergic pathways underlies the pathophysiology of depression in PD. The pathological changes in LC of PD patients are peculiar and can be differentiated from changes that occur in other neurodegenerative diseases such as schizophrenia. It was reported that simultaneous lesions of dopaminergic system, and LC causes metabolic dysfunction in the cerebral cortex and impairs cognitive functions in PD.
The Cerebellum And Parkinsonian Akinesia/rigidity
Parkinson’s disease is not a homogenous disease and has two predominant forms: akinesia and rigidity and prominent resting tremor . Akinesia can be defined as a delay or a failure in movement initiation , particularly for self-initiated movements. Functional neuroimaging studies using PET or blood oxygen level–dependent functional MRI frequently demonstrated increased activation in the cerebellum in patients with Parkinson’s disease during performance of various upper limb movements . For example, during externally or internally timed simple finger movements , motor timing , complex sequential movements , bimanual two-hand coordinated tasks or two different motor tasks simultaneously , patients with Parkinson’s disease OFF medication showed hyperactivation in the cerebellum.
Brain areas more activated in patients with Parkinson’s disease than in normal subjects during automatic execution of sequential movements. Modified fromWu and Hallett , with permission from Oxford University Press.
The neurodegenerative process in Parkinson’s disease begins several years before the onset of any clinical symptoms . The motor symptoms of Parkinson’s disease usually present after ?70% of dopaminergic neurons have degenerated . Presumably, the compensatory effect in the cerebellum and other brain regions accounts for delaying the onset of motor symptoms and preserving relatively normal function.
How Do Symptoms Progress And What Is The Outlook
The symptoms of PD tend to become gradually worse over time. However, the speed of progression varies greatly from person to person. When symptoms first begin, you may not need treatment when symptoms are relatively mild.
Most people with PD can expect to have some time of relatively mild symptoms. Then, when the symptoms become worse, they can expect several years of good or reasonable control of the symptoms with medication. But everyone is different and it is difficult to predict for an individual how quickly the disease will progress. Some people may only be slightly disabled 20 years after PD first begins, whereas others may be very disabled after 10 years.
Research into PD is active. For example, one main aim of research is to find medicines that prevent the damage to the affected cells, rather than just treating the symptoms, which is the main value of treatment at present. Further research on these chemicals continues. Research is underway using stem cell therapy to help treat PD. Other researchers are looking at alpha synuclein, a protein that gathers around the junction between nerve cells and is thought to affect the way messages are conducted between the brain and the nerves controlling movement.
Further reading and references
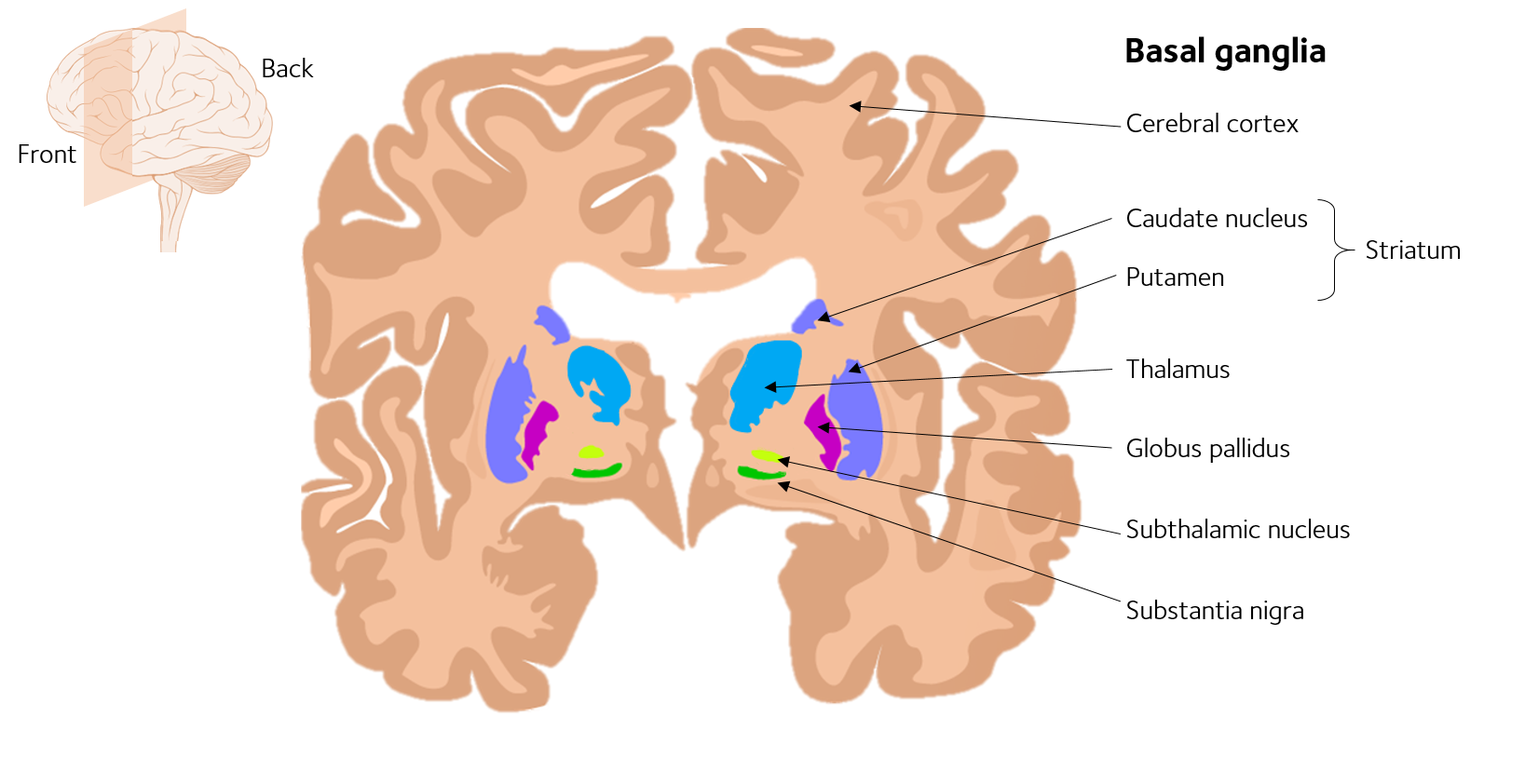
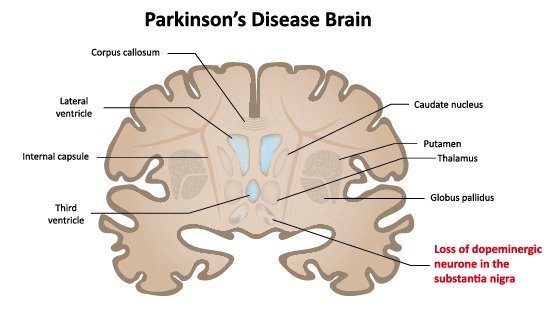
Changes In Basal Ganglia In Parkinson’s Disease
Since changes in the morphology of basal ganglia are noticed in bipolar and unipolar disorders also, it is essential to understand the changes in basal ganglia in PD for reliable diagnosis. Basal ganglia process the signals from the cortex for accurate execution of voluntary movements. Basal ganglia play a key role in cognitive functions, and lesion of basal ganglia causes impairment in cognitive functions. In fact, basal ganglia are the most affected brain area in PD. It was reported that there were two subtypes of PD and each type will affect basal ganglia in a different fashion. The subtypes include heterogeneous clinical phenotypes such as tremor dominant and postural instability/gait difficulty . PD patients had reduced fractional anisotropy within the substantia nigra and increased mean and radial diffusivity within the substantia nigra and globus pallidus. However, microstructural changes within the substantia nigra are severely affected in PIGD patients compared to TD. Reduction of the neuromelanin pigmentation, neuronal loss, and Lewy bodies are observed in the substantia nigra. In PD patients, basal ganglia undergo atrophy, which depends on severity and duration of the disease. In PD patients, loss of attenuation and length of the dendritic spines of medium-sized spiny neurons located in the striatum has been reported. Reduction in the volumes of the caudate nucleus and thalamus and white matter is observed in PD, which may be an early sign of disease progression.
Changes In Limbic System In Parkinson’s Disease
Influence of limbic structures on cognition was well documented. Atrophy of gray matter was observed in Parkinson’s patients with dementia. Dopamine dysfunction in the limbic system leads to change in the creativity and emotional dysfunction in PD patients. In amygdala, accessory cortical and central nuclei are affected more by PD, and cortical, accessory basal, and granular nuclei are least affected areas. Posterior cingulum is the important structure in the papez circuit which is involved in processing of episodic memory. It was reported that neuronal loss, gliosis, or demyelination in the white matter and metabolic changes occurs in the cingulum of PD patients. Change in the spontaneous resting-state neural activity is reported in prefrontal cortex, which was considered as a factor for cognitive decline in PD. Further, regional atrophy in the hippocampus contributes to impaired verbal learning memory and visuospatial processing.
Precautionary Measures With Parkinson’s Disease
Many people 50-60 years of age experience slight tremors, especially in the hands. This is not Parkinson’s disease. If you are experiencing other symptoms, or shake movements getting worse, you should consult a doctor.
It should be noted that some Parkinson’s patients will suffer from depression, and it is important that both doctor and family are aware of this possibility, since the treatment of depression will give more energy and capacity to cope with the other effects of the disease.
What Are The Treatments For Parkinson’s Disease
There is no cure for PD, and no treatment prevents the disease from progressing. However, treatments can usually ease symptoms.
- At first, you may not need any treatment when the symptoms are mild. A specialist may simply see you every now and then to monitor how the disease is progressing.
- A medicine that eases symptoms is usually started when symptoms become troublesome.
- Therapies such as physiotherapy, occupational therapy and speech therapy may also be useful as the disease progresses.
- Surgery may be an option for severe cases.
Changes In Brain Volume In Parkinson’s Disease
Strong correlation exists between brain size and cognitive functions. In PD patients, atrophy of the brain was observed in many cortical and subcortical areas, which contributes in decrease in the volume of the brain. Interestingly, it was reported that volume of the frontal lobe, temporoparietal junction, parietal lobe, insula, anterior cingulate cortex, basal ganglia, and thalamus increased in PD patients. Prefrontal lobe plays a crucial role in cognitive functions and in PD patients; loss of gray matter has been reported.
Changes In Hypothalamus In Parkinson’s Disease
Role of the hypothalamus in cognitive functions is well reported. Impairment of hypothalamic function was reported in PD patients. Neural degeneration was observed in all 13 nuclei of the hypothalamus with predominant degeneration in tuberomamillary nucleus and the lateral and posterior hypothalamic nuclei. Further, decrease in the levels of dopamine, serotonin, melanin, and hypocretin in the hypothalamus of PD patients. Development of sleep, endocrine, and autonomic disorders in PD may be due to dopamine malfunction in the hypothalamus. Sleep disorders may be due to loss of gray matter in hypothalamus as melatonin levels are associated with volume of gray matter. As hypothalamus functioning is affected in PD, the secondary degeneration may occur to the structures directly innervated by the hypothalamus such as striatum where the dopamine synthesis occurs. This further worsens the PD.
Changes In Glial Cells In Parkinson’s Disease
All glial cells can influence the cognitive functions. Structural changes occur in astrocytes in response to physiological and pathological conditions may influence the neurons through nonsynaptic communication with neurons. Altered neuroglial interaction may be the underlying cause for many neurological diseases including PD. Glial response in PD offers both beneficial and hazardous effects.
How Is Psp Different From Parkinson’s Disease
PSP is often misdiagnosed as Parkinson’s disease, especially early in the disorder, as they share many symptoms, including stiffness, movement difficulties, clumsiness, bradykinesia , and rigidity of muscles. The onset of both diseases is in late middle age. However, PSP progresses more rapidly than Parkinson’s disease.
- People with PSP usually stand exceptionally straight or occasionally tilt their heads backward . This is termed “axial rigidity.” Those with Parkinson’s disease usually bend forward.
- Problems with speech and swallowing are much more common and severe in PSP than in Parkinson’s disease and tend to show up earlier in the disease.
- Eye movements are abnormal in PSP but close to normal in Parkinson’s disease.
- Tremor is rare in PSP but very common in individuals with Parkinson’s disease.
Although individuals with Parkinson’s disease markedly benefit from the drug levodopa, people with PSP respond minimally and only briefly to this drug.
People with PSP show accumulation of the protein tau in affected brain cells, whereas people with Parkinson’s disease show accumulation of a different protein called alpha-synuclein.
Changes In Cerebellum In Parkinson’s Disease
Increased activity of the cerebellum was observed during cognitive tasks. The cerebellum has reciprocal connections with basal ganglia, and Parkinson’s-related morphological changes were observed in animal models and humans. These changes include significant contraction in the left cerebellum, decrease in the gray matter volume in the right quadrangular lobe. These changes may be induced by degeneration of dopaminergic neurons as the cerebellum receives dopaminergic projections from basal ganglia, and dopaminergic receptors were present in the cerebellum.