The Motor Network In Parkinsons Disease And Dystonia: Mechanisms Of Therapy
open to eligible people ages 21-75
This is an exploratory pilot study to identify neural correlates of specific motor signs in Parkinsons disease and dystonia, using a novel totally implanted neural interface that senses brain activity as well as delivering therapeutic stimulation. Parkinsons disease and isolated dystonia patients will be implanted unilaterally or bilaterally with a totally internalized bidirectional neural interface, Medtronic Summit RC+S. This study includes three populations: ten PD patients undergoing deep brain stimulation in the subthalamic nucleus , ten PD patients with a globus pallidus target and five dystonia patients. All groups will test a variety of strategies for feedback-controlled deep brain stimulation, and all patients will undergo a blinded, small pilot clinical trial of closed-loop stimulation for thirty days.
San Francisco, California
Recommended Reading: What Is An Essential Tremor Parkinson
Using Machine Learning In Research
The available treatments for Parkinsons disease to date are only partially effective and fail to markedly delay disease progression. Thus, there is growing interest in repurposing existing medications as an accelerated method of therapeutic development.
Such approved treatments, having already been rigorously tested in clinical trials, generally have established safety profiles.
Studies have suggested that people treated with certain medications, including immunosuppressants or those that widen the airways, called bronchodilators, have a lower risk of developing Parkinsons.
These findings prompted researchers based at the Université Paris-Saclay, in France, to use machine learning tools to automatically screen a large database of marketed therapies to detect those related to a lower risk of Parkinsons.
This study is part of a research effort aimed at identifying already-developed compounds associated with reduced risk, the researchers noted.
Data were collected from the French national health data system. A total of 40,760 incident Parkinsons patients were identified based on the details of at least one claim for an anti-Parkinsons medication from 2016 to 2018. A control group of 176,395 individuals of similar age, sex, and area of residence were included as a comparison.
Given that, the team assessed therapeutic exposure and related factors during the two years before the lag period to find associations to a reduced risk of developing Parkinsons disease.
Trials Registered On The Who Ictrp Database
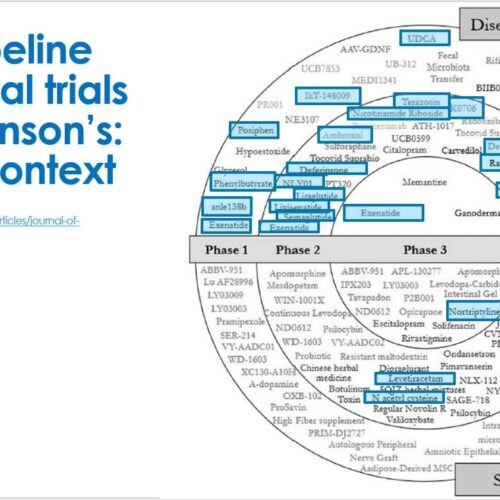
A total of 18 trials registered on WHO ICTRP list of other registries reached the final criteria for inclusion in our analysis. There are a large number of active clinical trials for PD on the WHO registries, but in most cases the information provided was lacking key details required for inclusion here . Of the included studies, there were 8 trials in Phase 1 or 1/2, 9 studies in Phase 2, and only 1 Phase 3 trial . Half of these studies were categorised as DMT.
Don’t Miss: Patient Teaching For Parkinson Disease
What Are Mesenchymal Stem Cells
Stem cells are the body’s raw materials â cells from which all other cells with specialized functions are created. Mesenchymal stem cells are adult stem cells that have self-renewal, immunomodulatory, anti-inflammatory, signaling, and differentiation properties. Mesenchymal stem cells , self renewal capacity is characterized by their ability to divide and develop into multiple specialized cell types present in a specific tissue or organ.
Mesenchymal stem cells can be sourced from a variety of tissue including adipose tissue , bone marrow, umbilical cord tissue, blood, liver, dental pulp, and skin.
MSCs are widely used in the treatment of various diseases due to their self-renewable, differentiation, anti-inflammatory, and immunomodulatory properties. In-vitro and in-vivo studies have supported the understanding mechanisms, safety, and efficacy of MSC therapy in clinical applications.
Parkinsons Research And Care
Description:
Learn how research helps shape treatments and identify new care strategies for managing Parkinsons symptoms. This program will also provide information on current research in Parkinsons and may include moderately scientific terms and concepts. The speakers are from the University of Arkansas for Medical Sciences .
Speakers:
Tuhin Virmani, MD, PhD, UAMS Health
Rohit Dhall, MD, MSPH, UAMS Health
Aditya Vikram Boddu, MD, UAMS Health
Hillary A. Williams, MD, UAMS Health
Host: Parkinsons Foundation
No information is available about the recording of this webinar.
Wednesday, November 16, 11am
Speaker: Kathy Lawrence, PT, Powerback Rehabilitation to You
Host: Parkinson Association of the Carolinas
A recording of the webinar will be posted to PAC Wellness Wednesday page
Wednesday, November 16, 1:30pm
Audience: Newly Diagnosed
Description:
If you or your loved one have been recently diagnosed with Parkinsons, chances are you are looking beyond your doctor for answers to your questions and for support on how to live well on this new journey!
Host: NeuroChallenge
No information is available about the recording of this webinar.
Thursday, November 17, 9am
Recommended Reading: What Causes Rigidity In Parkinson’s Disease
Biological Functions Of Hub Genes
To further investigate the biological functions of RPL3L, PLEK2, PYCRL, CD99P1, LOC100133130, MELK, LINC01101, and DLG3-AS1, GSEA was performed based on their ordered gene expression matrix. As shown in Figure 7, GSEA analyses revealed that RPL3L, PLEK2, PYCRL, CD99P1, LOC100133130, MELK, LINC01101, and DLG3-AS1 were mainly involved in glycosaminoglycan biosynthesis heparan sulfate/heparin, histidine metabolism, nicotine addiction, diabetes, protein export, taste transduction, fatty acid degradation, propanoate metabolism, endocrine, and other factorregulated calcium reabsorption, GABAergic synapse, steroid biosynthesis, synaptic vesicle cycle, carbohydrate digestion and absorption, proteasome, and asthma .
Figure 7. GSEA of hub genes: GSEA results for CD99P1 GSEA results for DLG3-AS1 GSEA results for LINC01101 GSEA results for LOC100133130 GSEA results for MELK GSEA results for PLEAK2 GSEA results for PYCRL GSEA results for RPL3L.
Aptinyx Focuses On Parkinsons Disease Cognitive Impairment
Aptinyx is amid a Phase II trial targeting one of the biggest unmet needs in Parkinson’s disease treatment: cognitive impairment and dementia.
In patients with PD, Lewy bodies and alpha-synuclein, which are implicated in the pathology of PD, can spread into the cortex, causing forms of cognitive impairment and dementia in a large percentage of patients, Kordower explains. Theres no current treatment, so any clinical trial that has some benefit is incredibly valuable, he says.
Aptinyxs NYX-458, which targets the N-methyl-D-aspartate receptor, has placebo-controlled Phase II results expected at the end of 2022 or early 2023. The trial lists eight primary endpoints, ranging from the incidence adverse events to reductions in scales of psychosis and suicidal ideation.
“There’s no current treatment, so any clinical trial that has some benefit is incredibly valuable.”
Jeffrey Kordower
Targeting NMDA is a good start, says Dr David Eidelberg, neurologist at the Feinstein Institutes for Medical Research. However, showing reduction in cognitive impairment may be more difficult in PD than in Alzheimers disease, where changes are typically more pronounced, he notes.
Nevertheless, experts are encouraged that a trial is taking aim at this substantial unmet need. According to GlobalData consensus forecasts, NYX-458 has expected peak sales of $323 million in 2028.
Don’t Miss: Glutathione Nasal Spray Parkinson’s
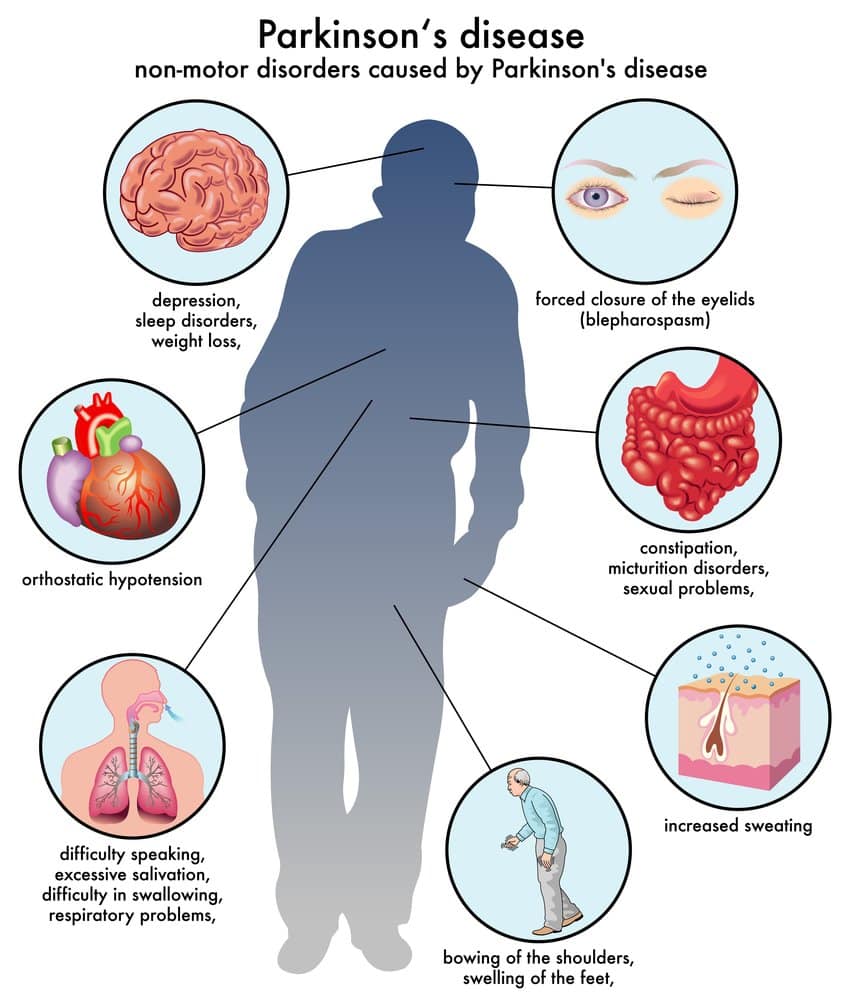
Understanding Sleep Problems To Prevent Parkinsons
Brain scans of people who experience RBD show increased inflammation the bodys natural response to injury, coming from immune cells in the brain called microglia. Research suggests that excessive levels of inflammation in the brain may cause damage to brain cells and play a role in the progression of Parkinsons.
The involvement of immune cells in Parkinsons has peaked researchers interest in the possibility of dialling down inflammation in the brain. And there is ongoing work through the Parkinsons Virtual Biotech to find and develop potential anti-inflammatory drugs.
Project Galaxy is focusing on developing molecules that can get into the brain and target a specific protein on the surface of microglia that has been shown to be present at much higher levels in the brains of people with Parkinsons than in people without the condition. This could pave the way for a future treatment to slow or stop the progression of the condition.
Targeting inflammation at the earliest stages of Parkinsons may also be important when aiming to slow the loss of cells and delay the onset and progression of symptoms. While identifying Parkinsons early is challenging, doing so opens the door to treating the condition before symptoms become problematic and potentially even preventing people from developing Parkinsons.
Over The Counter & Complementary Therapies
People with Parkinsons who seek relief from their symptoms may decide to explore complementary therapies, which can support or complement traditional medicine. While there are many kinds of complementary medicine, this section focuses on herbs, vitamins and supplements.
Page reviewed by Dr. Chauncey Spears, Clinical Assistant Professor and Dr. Amelia Heston, Movement Disorders Fellow at the University of Michigan.
Recommended Reading: Do Dogs Get Parkinson’s Disease
A Decade In The Making
Nearly one million people are living with Parkinsons disease in the United States alone, according to the Parkinsons Foundation. About 60,000 people are diagnosed with Parkinsons each year, and that number is expected to rise to 1.2 million by the end of this decade. It is the fastest rising neurological disorder in the world, with the global number of diagnosed people doubling from 3 to 6 million people between 1990 and 2015. If this development continues, the number of cases will again have doubled by 2040.
If the rate at which Parkinsons growth continues, were going to outgrow the capacity to be able to handle all of the consequences of letting a chronic neurodegenerative disease go unchecked, Michael S. Okun, a neurologist at the University of Florida and one of the worlds leading Parkinsons scientists, told The Daily Beast.
After decades of research, what we know so far is that Parkinsons is caused when dopamine-producing nerve cells in the brain die too fast. Often called the happy hormone, dopamine is critical for relaying signals from the brain that give orders of movement to different body parts. A dearth of dopamine will cause tremors and slowed movement. These symptoms only worsen over time, and make it extremely difficult to do even the simplest activities in the late stages of the illness.
And Parkinsons can lead to cognitive effects as well, such as short-term memory loss, difficulties with staying focused, and challenges with impulse control.
Medication For Parkinsons Disease
Once the doctor diagnoses Parkinsons disease, the next decision is whether a patient should receive medication, which depends on the following:
-
The degree of functional impairment
-
The degree of cognitive impairment
-
Ability to tolerate antiparkinsonian medication
-
The advice of the attending doctor
No two patients react the same way to a given drug, therefore, it takes time and patience to find an appropriate medication and dosage to alleviate symptoms.
Read Also: What’s Good For Parkinson’s
Enfermedad De Parkinson Lo Basico
Seminario web mensual Todo sobre el Parkinson.
Presentadora: Natalie Diaz, MD, Pacific Neuroscience Institute , Santa Monica, CA
Anfitrión: Pacific Neuroscience Institute , Santa Monica, CA
No es necesario registrarse.
Webinar ID: 821 8048 1494
Telephone: 669-900-6833
Para preguntas o más información, comuníquese con Giselle, teléfono 310-582-7433,
La grabación del seminario web se publicará en la sección Trastornos del movimiento de la página de seminarios web de Salud y Bienestar de PNI
Wednesday, November 9, 10am
A Promising Novel Treatment
Subramanian: One of the things that caught my eye was a study of the GLP-1 agonist liraglutide, which is a novel treatment in Parkinson’s disease. It’s something that’s been approved, I believe, in diabetes and obesity treatment. There’s been a lot of interest in the GLP-1, which are the glucagon-like peptide 1 drugs, the agonists of those receptors.
Basically, Dr Michele Tagliati, who’s a colleague of mine just up the street at Cedars-Sinai, has put a lot of passion, interest, and focus into studying liraglutide. This was a single-center, randomized, double-blind, placebo-controlled trial. In addition to regular medicines, they gave once-daily, self-administered injections of liraglutide at 1.2 or 1.8 mg as tolerated, vs placebo, for 52 weeks after titration. They looked at some outcome measures that included the Movement Disorders Society Unified Parkinson’s Disease Rating Scale part three, which is the motor measure. They also looked at the nonmotor symptoms scale, as well as the Mattis Dementia Rating Scale at week 54. There were 63 patients randomized, 43 to liraglutide and 21 to placebo.
The bottom line was interesting. There was improvement in the clinical features of Parkinson’s disease, including nonmotor symptoms and activities of daily living. But it didn’t look like there were improvements in terms of the MDS-UPDRS or the Mattis Dementia Rating Scale.
Recommended Reading: Does Parkinson Disease Affect Your Vision
Treatments In Phase I Trials
As Parkinsons researchers work toward more precision medicines, they benefit from a wide range of therapeutic categories & tools to experiment with. Therapeutic categories refer to all the different categories of research focuses, like stem cells, antioxidants, and gene targeting, for example. With trials spanning more than 15 therapeutics categories in the Phase I pipeline, it has a broad range of focuses. Making discoveries in more therapeutic categories means we can one day provide access to personalized treatment solutions based on the specific causes and symptom presentation of each person living with Parkinsons.
There are 50 treatments in Phase I trials this year with about a 50/50 split between symptom-relieving products and disease-modifying treatments. Half of the treatments in Phase I are new discoveries as the result of pathfinding research. This is when researchers use insights from existing medications to seek out related products that warrant exploration.
Component #2 A Neuroprotective Agent
Once a drug or a treatment has been determined to slow down the progression of Parkinsons, it will be necessary to protect the remaining cells and provide a nurturing environment for the third part of the cure .
Neuroprotection is the area of research that has had the most attention over the years. Drug companies have employed vast resources in this area in the hope of discovering a treatment which will work across conditions , and thus provide them with tremendous profits. Unfortunately, conditions of the brain have proven to be a lot more complicated than first perceived and cross-condition therapies seem unlikely as we move towards greater stratification and personalisation of disease and treatment, respectively.
But there has been the hint of a potential neuroprotective effect in one class of drugs for Parkinsons: GLP-1R agonists.
Neuroprotective approach: GLP-1R agonists
Exenatide is a glucagon like peptide-1 receptor agonist. This is a class of drug that has traditionally been used for treating diabetes, but has recently been repurposed for Parkinsons.
After multiple studies suggested neuroprotective properties in models of Parkinsons, a clinical trial program was intiated, and in 2017, a Phase II Exenatide trial reported the stablisation of Parkinsons motor features over the course of the 48 week trial .
Reduction in motor scores in Exenatide group. Source: Lancet
In late 2019, we saw the initiation of a Phase III clinical trial for .
Read Also: Swimming And Parkinson’s Disease
Loud Clear & Controlled: Managing Speech & Voice Issues In Pd
Description:
Learn the common speech and voice issues in Parkinsons and why they occur, the Bread & Butter exercises everyone with PD should be doing daily, why its never too early to start doing speech and voice exercise, and how to get started and stay diligent with regular speech and voice exercise. Bring your questions about speech and voice PD issues.
Speaker: Sarah Awde, SLP, Get Loud Therapy, Ontario, Canada
Host: American Parkinson Disease Association Northwest
Screening Of Key Modules And Genes Based On Wgcna
We extracted the expression data of differentially expressed genes in samples of persons with PD for co-expression analysis. First, the soft threshold was selected for subsequent co-expression network construction . The principle was to make the constructed network more in line with the characteristics of the scale-free network. The R-square was set as 0.85 . WGCNA was used to construct the co-expression network module and visually display the modules’ gene correlation. Fourteen co-expression modules were obtained, and the number of genes in each module was at least 50. The results were displayed in a hierarchical clustering diagram . Then, a heat map was mapped on module-trait relationships according to the Spearman correlation coefficient to evaluate the association between each module and the disease . Two modules MEdarkgrey and MEdarkorange had high association with PD and were selected as PD-related modules . The MEdarkgrey and MEdarkorange modules were positively correlated with PD, 5,801, and 7,763 genes, respectively. Target genes were obtained by intersecting DEGs with key genes based on WGCNA screening .
Read Also: Does Parkinson’s Cause Cognitive Impairment
Amneal Tests A New Formulation
AmnealPharmaceuticals plans to report Phase III safety results for IPX-203, a reformulation of the common generic PD treatment combination of carbidopa and levodopa that could reduce symptom fluctuations. The company said the Phase III, open-label extension study will have results available by the end of the second quarter of 2022.
CD/LD can lead to troughs and spikes of plasma levels that generate side-effects like dyskinesia, Kordower explains. A new extended-release version of CD/LD could smooth out these drops, he notes.
If approved, IPX-203 will join several other marketed reformulations of CD/LD. Amneals own extended-release capsule Rytary, Schwarz Pharmas orally disintegrating tablet Parcopa, and AbbVie’s enteral suspension Duopa all have FDA approval in PD. A GlobalData consensus forecasts pegs peak IPX-203 sales at $127 million in 2028.
In a separate, placebo-controlled Phase III trial , IPX-203 resulted in 0.53 more hours of ON time than immediate-release CD/LD after seven weeks . Earlier, a six-week Phase II trial of IPX-203 reported no serious treatment-emergent adverse events among the 26 patients enrolled. Experts say the long-term safety data will be key in determining IPX-203s place among CD/LD formulations.
Establishment Of A Diagnostic Nomogram For Pd
A diagnostic nomogram was established based on the hub genes by using the rms package in R software. The receiver operating characteristic curve was used to investigate the efficiency of this diagnostic model. The area under curve > 0.7 was considered significant. Additional expression profile data of PD was acquired from the GEO database to validate the nomogram.
Also Check: Does Jason Beghe Have Parkinson’s Disease
New York November 7 2022
Anavex Life Sciences Corp. , a clinical-stage biopharmaceutical company developing differentiated therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders including Alzheimers disease, Parkinsons disease, Rett syndrome and other central nervous system disorders, today reports the U.S. Food and Drug Administrations has granted Orphan Drug Designation to ANAVEX®2-73 for the treatment of Fragile X syndrome.
Fragile X syndrome is the most common form of inherited intellectual disability and the most frequent single gene cause of autism spectrum disorder with an estimated population of approximately 62,500 in the US and 1,088,500 worldwide. At present, there is no approved treatment for Fragile X syndrome.
The Orphan Drug Designation highlights the potential to expand the therapeutic profile of ANAVEX®2-73 into the largest portion of autism spectrum disorder, Fragile X syndrome, said Christopher U Missling, PhD, President and Chief Executive Officer of Anavex. We look forward to working with the Fragile X syndrome community to rapidly advance ANAVEX®2-73 as a potential treatment for Fragile X syndrome while we continue to expand late-stage clinical investigation of ANAVEX®2-73 as part of its precision medicineplatform technology for both neurodevelopmental and neurodegenerative indications.
About Fragile X Syndrome and Autism Spectrum Disorder
About Anavex Life Sciences Corp.
Forward-Looking Statements
For Further Information:
