Parkinson Disease Shown To Induce Immune Imbalance In The Blood Indicating Possible Benefit Of Immune Modulation
Researchers uncovered that patients with Parkinson disease could benefit from immune modulation as an alternative treatment, due to the condition’s influence on immune imbalance, according to study findings.
Researchers uncovered that patients with Parkinson disease could benefit from immune modulation as an alternative treatment, due to the condition’s influence on immune imbalance, according to findings of a study published in the journal Movement Disorders.
PD is a multisystem disease in which both the central and peripheral nervous systems are affected. The disease is characterized by the slow degeneration of the neurons in the brain due to the abnormal accumulation of a protein called ?-synuclein. While it is primarily seen as a brain disorder, the behavior of immune cells in the blood of affected patients is starkly different than in those without the disease. This evidence reveals a potential extension to usual research of PD-related changes on immune response, indicating a further impact on peripheral immune cells, such as monocytes, in addition to microglia in the brain.
Researchers from the Department of Biomedicine at Aarhus University sought to investigate this understudied aspect of PD-related changes in peripheral immune cells by examining their responsiveness to stimulation and their ability to release immunomodulatory molecules, which is linked to consequences for PD progression:
Reference
First Direct Evidence That Abnormal Protein In Parkinson’s Disease Triggers Immune Response
- Date:
- Columbia University Medical Center
- Summary:
- Researchers have found the first direct evidence that autoimmunity plays a role in Parkinson’s disease, suggesting that immunosuppressants might play a role in treatment.
Researchers have found the first direct evidence that autoimmunity — in which the immune system attacks the body’s own tissues — plays a role in Parkinson’s disease, the neurodegenerative movement disorder. The findings raise the possibility that the death of neurons in Parkinson’s could be prevented by therapies that dampen the immune response.
The study, led by scientists at Columbia University Medical Center and the La Jolla Institute for Allergy and Immunology, was published today in Nature.
“The idea that a malfunctioning immune system contributes to Parkinson’s dates back almost 100 years,” said study co-leader David Sulzer, PhD, professor of neurobiology at CUMC. “But until now, no one has been able to connect the dots. Our findings show that two fragments of alpha-synuclein, a protein that accumulates in the brain cells of people with Parkinson’s, can activate the T cells involved in autoimmune attacks.
The Sulzer and Sette labs are now analyzing these responses in additional patients, and are working to identify the molecular steps that lead to the autoimmune response in animal and cellular models.
Story Source:
First Direct Evidence That Abnormal Protein In Parkinsons Disease Triggers Immune Response
New York, NY —Researchers have found the first direct evidence that autoimmunity—in which the immune system attacks the body’s own tissues—plays a role in Parkinson’s disease, the neurodegenerative movement disorder. The findings raise the possibility that the death of neurons in Parkinson’s could be prevented by therapies that dampen the immune response.
The study, led by scientists at Columbia University Irving Medical Center and the La Jolla Institute for Allergy and Immunology, was published today in Nature.
“The idea that a malfunctioning immune system contributes to Parkinson’s dates back almost 100 years,” said study co-leader David Sulzer, PhD, professor of neurobiology . “But until now, no one has been able to connect the dots. Our findings show that two fragments of alpha-synuclein, a protein that accumulates in the brain cells of people with Parkinson’s, can activate the T cells involved in autoimmune attacks.
“It remains to be seen whether the immune response to alpha-synuclein is an initial cause of Parkinson’s or if it contributes to neuronal death and worsening symptoms after the onset of the disease,” said study co-leader Alessandro Sette, Dr. Biol. Sci., professor in the Center for Infectious Disease at La Jolla Institute for Allergy and Immunology in La Jolla, Calif. “These findings, however, could provide a much-needed diagnostic test for Parkinson’s disease and could help us to identify individuals at risk or in the early stages of the disease.”
###
New Research Gives Further Evidence That Autoimmunity Plays A Role In Parkinsons Disease
Member for
ScienMag
A new study co-led by scientists at the La Jolla Institute for Immunology adds increasing evidence that Parkinson’s disease is partly an autoimmune disease. In fact, the researchers report that signs of autoimmunity can appear in Parkinson’s disease patients years before their official diagnosis. The research could make it possible to someday detect Parkinson’s disease before the onset of debilitating motor symptoms–and potentially intervene with therapies to slow the disease progression. The Parkinson’s Foundation supports this research.
Evidence In Recent Years Suggests That Parkinsons Disease Could Be An Autoimmune Disease
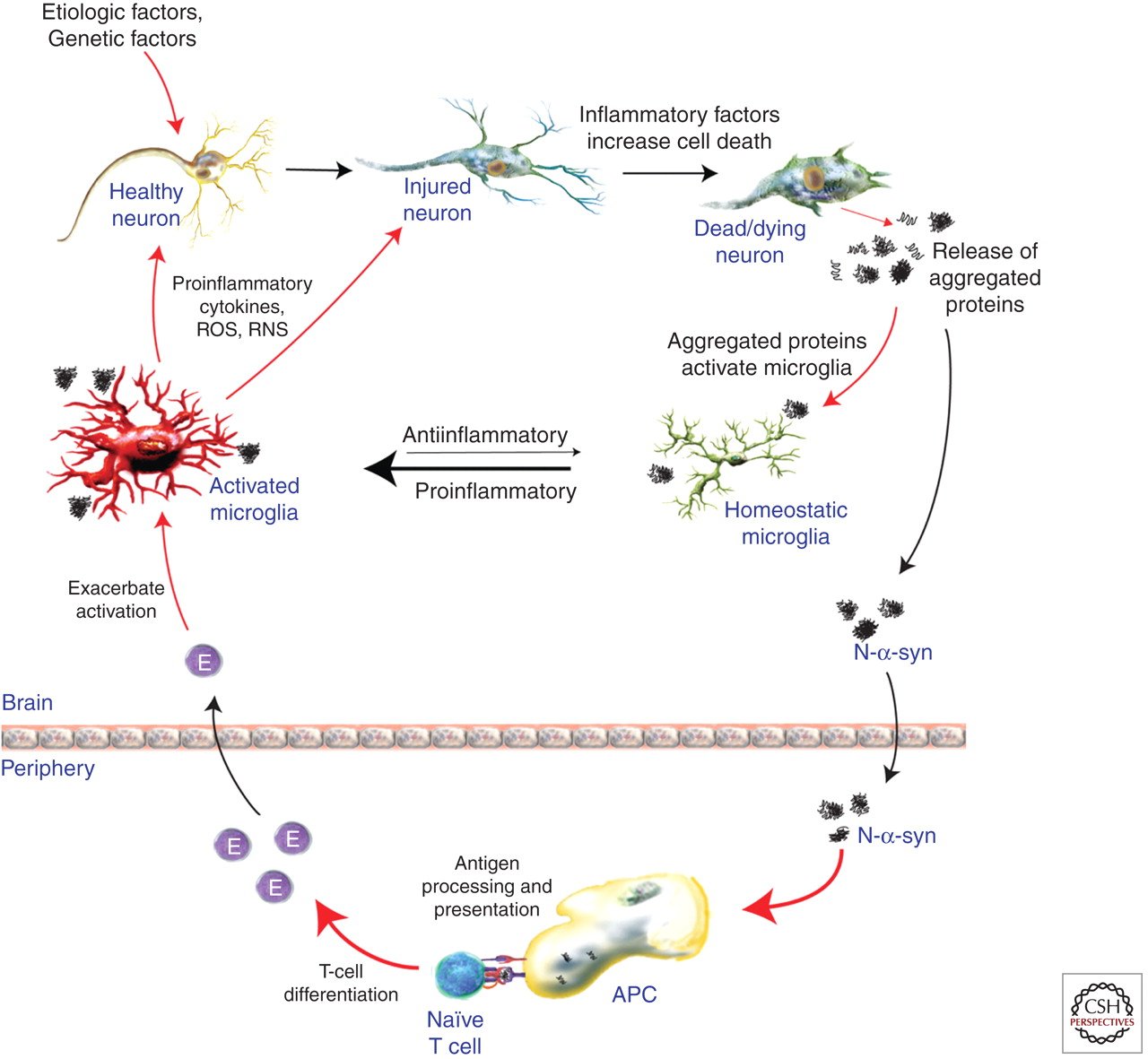
For decades, scientists and doctors alike have known that inflammation causes changes in the brains of Parkinson’s disease patients. However, it is only in recent years that they have come to understand that this inflammation is part of the cause of the progressive nature of PD and is not merely a result of the disease.
In 2018, a study conducted by researchers in Germany found further evidence supporting the idea that Parkinson’s could in fact be an autoimmune disease. By examining a stem cell model, they demonstrated how immune cells attacked dopamine-producing cells taken from PD patients but not from people who did not have PD.
Dopamine is a neurotransmitter made in the brain that supports a number of functions, including those that deal with emotions, reward, pleasure, and movement control. In Parkinson’s disease, the cells that make dopamine, the midbrain neurons, die off.
As more and more dopamine cells die over time, the neurotransmitter levels decrease, leading to a number of symptoms including tremors, balance issues, muscle rigidity, and slow body movement. Swallowing and speech issues also occur, as well as several non-movement symptoms, such as distorted or lost sense of smell, sleep disturbances, fatigue, confusion, and anxiety.
It is not yet clear what exactly causes this midbrain neuron death.
Decreased Risk Of Parkinsons Disease After Rheumatoid Arthritis Diagnosis: A Nested Case
Article type: Research Article
Affiliations: Center for Neurodegenerative Science, Van Andel Institute, Grand Rapids, MI, USA | Department of Clinical Sciences Lund, Rheumatology, Lund University, Lund, Sweden | Center for Epigenetics, Van Andel Institute, Grand Rapids, MI, USA | Institute of Biomedicine, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden | Department of Psychiatry West, College of Human Medicine, Michigan State University, Grand Rapids, MI, USA
Correspondence: Correspondence to: Lena Brundin, Center for Neurodegenerative Science, Van Andel Institute, 333 Bostwick Ave., N.E., Grand Rapids, MI 49503, USA. Tel.: +1 616 234 5000; E-mail: .
Note: Shared last authors.
Keywords: Rheumatoid arthritis, Parkinson’s disease, autoimmune disease, anti-inflammatory
DOI: 10.3233/JPD-202418
Journal: Journal of Parkinson’s Disease, vol. 11, no. 2, pp. 821-832, 2021
Abstract
Potential For Ibogaine In The Treatment Of Parkinsons And Autoimmune Diseases
Ibogaine is an alkaloid derived from the African shrub Tabernanthe iboga. Aside from its psychoactive and anti-addiction properties, it has beneficial effects on certain neurotrophic factors and receptors in the brain. Targeting them has shown great potential for the future therapy of neurodegenerative and neuroinflammatory conditions such as Parkinson’s.
Parkinson’s disease is a crippling neurological disorder that affects more than 1 million people in the US. The number of people with the condition is expected to increase by more than 60% in the next 20 years . Currently, its cause is unknown but new evidence suggests that its main pathogenetic mechanism might be related to an autoimmune reaction that is present even before the onset of the first symptoms.
Autoimmune diseases are debilitating conditions that affect about 5% of the population. Currently, there is no cure for Parkinson’s or other autoimmune disorders. The treatment options aim at reducing symptoms, preventing complications, and prolonging life.
Data From Animal Models Support A Role For T Cells In Disease Pathogenesis
Although animal models have limitations in recapitulating PD, they are useful tools for genetic manipulation and identifying features of disease pathology. A range of models for studying PD have been developed, employing toxins or genetic mutations that recapitulate certain aspects of the disease. Most of the models highlight a causative role for infiltrating T cells in propagating neurodegeneration.
Toxin Models of PD
Intracerebral injections of the toxin 6-hydroxy-dopamine into the midbrain of mice induces degeneration of dopamine and noradrenaline neurons , which are rendered vulnerable to the drug because they express the dopamine transporter, DAT, that accumulates the toxin. Once in the neuronal cytosol, 6-OHDA mediates oxidative stress-related cytotoxicity . The subsequent acute neurodegeneration is manifested in motor deficits in mice and, if injected unilaterally, in rotational motions. 6-OHDA treated mice show IgG leakage, indicative of a leaky BBB, as well as T and B cell infiltration around CNS blood vessels . Treg cells are significantly decreased in the periphery of 6-OHDA treated rats, which are reported to not show marked T cell infiltration into the midbrain .
Viral Overexpression of ?-Syn to Model PD
Is Parkinsons An Autoimmune Disease What Does The Current Research Say
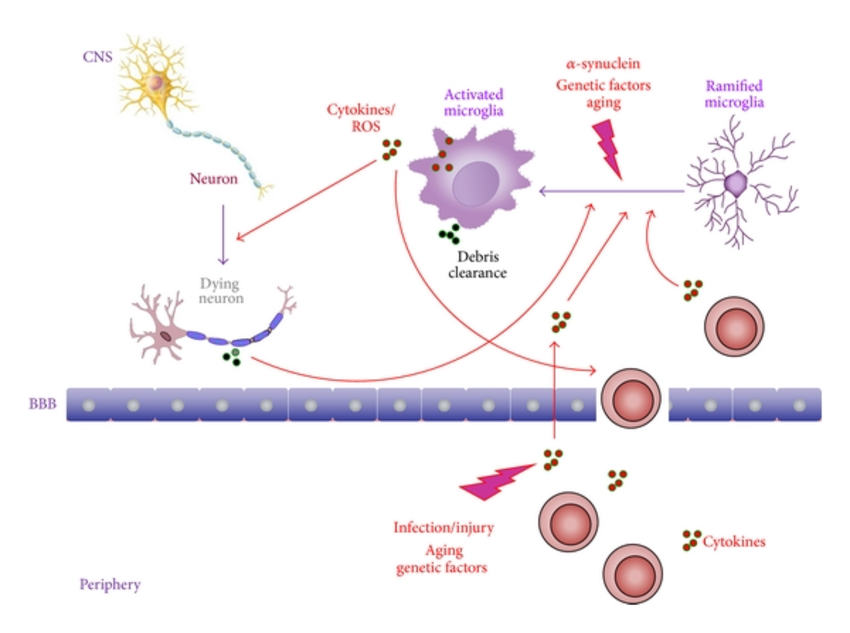
Parkinson’s disease is a long-term neurodegenerative disease that affects movement. The disease, which affects around half a million Americans, currently has no cure.
In honor of World Parkinson’s Day, we wanted to shine a light on groundbreaking research that has the potential to one day lead to a cure!
What does recent research say about Parkinson’s disease? Is Parkinson’s an autoimmune disease? What could this mean for the future of Parkinson’s treatments?
We break it down in our article!
Autoimmunity In Neurodegenerative Diseases And Its Relevance To Pd Figure 1
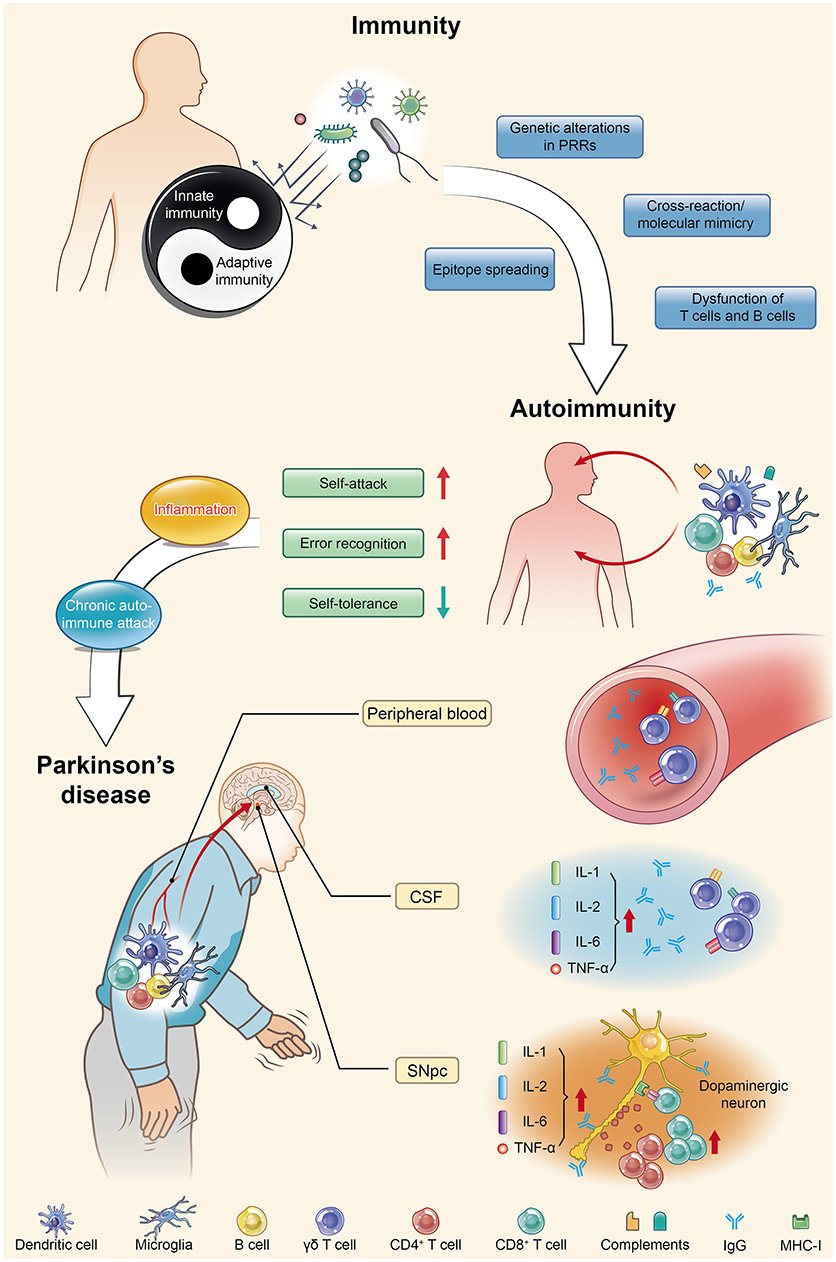
Parkinson’s disease is actually an autoimmune disease. Autoimmunity occurs when immune homeostasis is broken by several main mechanisms shown in this figure, which directly result in an increase in error recognition and self-attack and a decrease in self-tolerance to autoantigens. Regarding PD, chronic autoimmune attack is not only its pathogenesis but also always involved throughout the entire disease process. Inflammation is the first step of this attack, with the subsequent participation of various immune cells and immunoglobulins they produce, ultimately leading to the death of dopaminergic neurons. PRRs, pattern recognition receptors; CSF, cerebrospinal fluid; SNpc, substantia nigra pars compacta; IL, interleukin; TNF, tumor necrosis factor.
The Connection With Innate Immunity And Antigen Presenting Cells
Microglia are the primary immune cells of the CNS and act as both resident phagocytic and antigen presenting cell to provide an active immune defense . Microgliosis is characterized by microglia proliferation, change in morphological state from ramified to amoebic, and the presence of several inflammatory markers, such as CD68 . Microglial activation is an important potential player in PD-associated inflammation and immune reactivity. Neurons do not express Class II, while microglia are MHC Class II expressing antigen-presenting cells. MHC Class II present peptides derived from extracellular proteins , and cells of the monocyte lineage such as microglia are particularly apt at internalizing aggregated proteins and presenting peptides derived from exogenous proteins in the context of class II MHC molecules. They therefore represent a strong candidate for MHC class II restricted presentation of ?-syn peptides to T cells.
Parkinsons Autoimmune Disorders May Share Genetic Common Ground
June 12, 2017
NEW YORK —Parkinson’s disease and some autoimmune diseases may have genetic risk factors in common, raising the possibility that the immune system may influence Parkinson’s disease pathogenesis, new research suggests.
You Might Also Like
Previous research has pointed to an association between inflammation and neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease. Clinical studies have also found a lowered risk of PD in people who regularly use nonsteroidal anti-inflammatory drugs.
However, studies have yielded conflicting results with respect to a potential immune component in Parkinson’s disease, Dr. Sharma says.
To investigate, the researchers used existing data sets from genome-wide association studies in PD and from seven autoimmune disorders .
Using an approach called conjunctional false discovery rate analysis, the investigators analyzed GWAS data involving more than 138,000 individuals of European ancestry in search of pleiotropic gene loci, or markers, associated with both PD and the autoimmune disorders.
The analysis identified 17 novel “shared susceptibility loci” with overlap between Parkinson’s disease and the seven autoimmune diseases, the researchers report in JAMA Neurology, online June 5.1
Parkinsons: Autoimmune Attack May Start Years Before Diagnosis
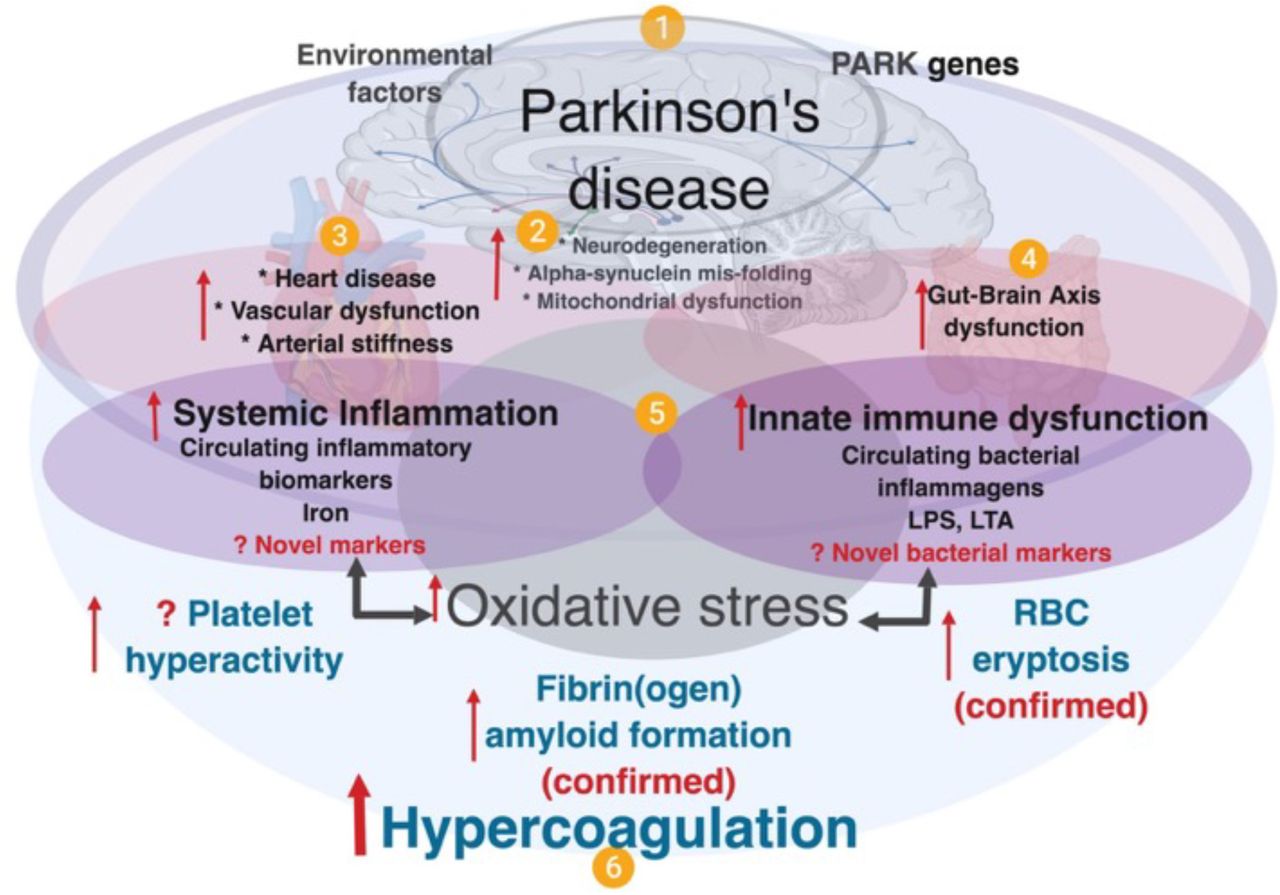
A new study adds to evidence that autoimmunity plays a role in the development of Parkinson’s disease. The research also offers hope that early preventive treatment could offset the damage.
Parkinson’s disease is a chronic, progressive disorder. Its characteristics tend to include tremor, rigidity, slowness of movement, and impaired balance.
Around 1 million people in the United States and 10 million people throughout the world have the disease.
Parkinson’s results from a loss of nerve cells in a part of the brain called the substantia nigra. These cells produce dopamine, a chemical messenger, or neurotransmitter, involved in controlling movement.
Most people with Parkinson’s are older than 50 when they receive the diagnosis, but some develop motor symptoms, involving problems with muscle control, at an earlier age.
Years before motor symptoms arise, other symptoms of Parkinson’s can appear, including a reduced sense of smell, constipation, mood changes, and REM sleep behavior disorder, which involves physically acting out dreams.
The existence of these prediagnostic symptoms suggests that damage to dopamine-producing nerve cells begins long before the person experiences trouble with movement.
A new study — spearheaded by researchers from the La Jolla Institute for Immunology , in California — adds to evidence that the immune system may be responsible for the damage to nerve cells.
What Is Autoimmunity How Does It Link To Parkinsons Disease
The immune system is a network of biological processes that protects the body from illness. It is able to detect and respond to a number of pathogens, ranging from viruses to parasites, from cancer cells to even foreign objects such as wooden splinters, and distinguish them from the body’s own healthy tissue. When the immune system makes a mistake and misidentifies and attacks the body’s own organs or tissues, this is called autoimmunity, and can lead or develop into an autoimmune disease.
Attack By Own Immune System May Kill Neurons In Parkinsons
The cause of neuronal death in Parkinson’s disease is still unknown, but a new study proposes that neurons may be mistaken for foreign invaders and killed by the person’s own immune system, similar to the way autoimmune diseases like type I diabetes, celiac disease, and multiple sclerosis attack the body’s cells. The study was published April 16, 2014, in Nature Communications.
“This is a new, and likely controversial, idea in Parkinson’s disease; but if true, it could lead to new ways to prevent neuronal death in Parkinson’s that resemble treatments for autoimmune diseases,” said the study’s senior author, David Sulzer, PhD, professor of neurobiology in the departments of psychiatry, neurology, and pharmacology at Columbia University College of Physicians & Surgeons.
The new hypothesis about Parkinson’s emerges from other findings in the study that overturn a deep-seated assumption about neurons and the immune system.
For decades, neurobiologists have thought that neurons are protected from attacks from the immune system, in part, because they do not display antigens on their cell surfaces. Most cells, if infected by virus or bacteria, will display bits of the microbe on their outer surface. When the immune system recognizes the foreign antigens, T cells attack and kill the cells. Because scientists thought that neurons did not display antigens, they also thought that the neurons were exempt from T-cell attacks.
Scientists Link Immune Cells To Parkinson’s Disease Onset
- Date:
- La Jolla Institute for Immunology
- Summary:
- A new study adds increasing evidence that Parkinson’s disease is partly an autoimmune disease. In fact, the researchers report that signs of autoimmunity can appear in Parkinson’s disease patients years before their official diagnosis.
A new study co-led by scientists at the La Jolla Institute for Immunology adds increasing evidence that Parkinson’s disease is partly an autoimmune disease. In fact, the researchers report that signs of autoimmunity can appear in Parkinson’s disease patients years before their official diagnosis.
The research could make it possible to someday detect Parkinson’s disease before the onset of debilitating motor symptoms — and potentially intervene with therapies to slow the disease progression.
The study, published in the April 20, 2020, issue of Nature Communications, was co-led by LJI professor Alessandro Sette, Dr. Biol. Sci, and Professor David Sulzer, Ph.D., of the Columbia University Medical Center.
Scientists have long known that clumps of a damaged protein called alpha-synuclein build up in the dopamine-producing brain cells of patients with Parkinson’s disease. These clumps eventually lead to cell death, causing motor symptoms and cognitive decline.
The researchers hope to study more Parkinson’s patients and follow them over longer time periods to better understand how T cell reactivity changes as the disease progresses.
Story Source:
Genes Connect Parkinsons Disease To Autoimmune Diseases

July 3, 2017
A growing body of research has revealed a link between Parkinson’s disease and the immune system. Scientists now suspect that inflammation of the microglia plays a role in the degeneration of the dopaminergic neurons affecting Parkinson’s disease. Researchers have also identified a few genetic comorbidities between high-risk Parkinson’s disease loci and immune-related genes. Specifically, they have found a strong pleiotropic enrichment between Parkinson’s disease and Crohn’s disease, suggesting that there may be a common pathogenic link between the two phenotypes.
You Might Also Like
New research by Aree Witoelar, PhD, postdoctoral researcher at the University of Oslo in Norway, and colleagues provides a novel mechanistic insight into Parkinson’s disease and autoimmune diseases. The research, online June 5 in JAMA Neurology, identifies a common genetic pathway between the different phenotypes. “We found moderate polygenic pleiotropic enrichment between and ulcerative colitis or RA , whereas genetic enrichment with Type 1 diabetes, celiac disease, psoriasis and multiple sclerosis was weak,” write the authors.1
Parkinson’s Is Partly An Autoimmune Disease Study Finds
Researchers have found the first direct evidence that autoimmunity—in which the immune system attacks the body’s own tissues—plays a role in Parkinson’s disease, the neurodegenerative movement disorder. The findings raise the possibility that the death of neurons in Parkinson’s could be prevented by therapies that dampen the immune response.
The study, led by scientists at Columbia University Medical Center and the La Jolla Institute for Allergy and Immunology, was published today in Nature.
“The idea that a malfunctioning immune system contributes to Parkinson’s dates back almost 100 years,” said study co-leader David Sulzer, PhD, professor of neurobiology at CUMC. “But until now, no one has been able to connect the dots. Our findings show that two fragments of alpha-synuclein, a protein that accumulates in the brain cells of people with Parkinson’s, can activate the T cells involved in autoimmune attacks.
“It remains to be seen whether the immune response to alpha-synuclein is an initial cause of Parkinson’s, or if it contributes to neuronal death and worsening symptoms after the onset of the disease,” said study co-leader Alessandro Sette, Dr. Biol. Sci., professor in the Center for Infectious Disease at La Jolla Institute for Allergy and Immunology in La Jolla, Calif. “These findings, however, could provide a much-needed diagnostic test for Parkinson’s disease, and could help us to identify individuals at risk or in the early stages of the disease.”
Explore further
The Connection Between The Immune System & Parkinson’s
In the recent study, researchers from Columbia University Medical Center and the La Jolla Institute for Allergy and Immunology collected blood samples from 67 people with PD and 36 age-matched healthy control subjects. The blood was then exposed to fragments of alpha-synuclein and other proteins that are found in neurons.
In the healthy controls, there was little immune activity detected, but in the blood samples from people with PD, there was a significant response. Researchers believe that the T-cells in people with PD have been primed to recognize alpha-synuclein from past exposure – the exposure being the presence of Lewy bodies in the brain. The immune response seen in the study subjects with PD was associated with a common gene variant that is known to affect the immune system, a gene variant that is common in people with PD.
Researchers hypothesize that autoimmunity in people with PD may develop when the neurons are no longer able to rid themselves of the abnormal accumulations of alpha-synuclein. In normal, healthy cells, old or damaged proteins are broken down and eliminated, but this process is not functioning properly in PD. When alpha-synuclein begins to form clumps, the immune system may mistakenly see it as a pathogen that needs to be attacked.1,2
Drugs And Medication Used To Treat Parkinsons Disease
A number of different drugs can be used to treat Parkinson’s.
Levodopa
Levodopa is the most common treatment for Parkinson’s. It helps to replenish dopamine.
About 75 percent of cases respond to levodopa, but not all symptoms are improved. Levodopa is generally given with carbidopa.
Carbidopa delays the breakdown of levodopa which in turn increases the availability of levodopa at the blood-brain barrier.
Dopamine agonists
Dopamine agonists can imitate the action of dopamine in the brain. They’re less effective than levodopa, but they can be useful as bridge medications when levodopa is less effective.
Drugs in this class include bromocriptine, pramipexole, and ropinirole.
Anticholinergics
Anticholinergics are used to block the parasympathetic nervous system. They can help with rigidity.
Benztropine and trihexyphenidyl are anticholinergics used to treat Parkinson’s.
Amantadine
Amantadine can be used along with carbidopa-levodopa. It’s a glutamate-blocking drug . It offers short-term relief for the involuntary movements that can be a side effect of levodopa.
COMT inhibitors
Catechol O-methyltransferase inhibitors prolong the effect of levodopa. Entacapone and tolcapone are examples of COMT inhibitors.
Tolcapone can cause liver damage. It’s usually saved for people who do not respond to other therapies.
Ectacapone does not cause liver damage.
Stalevo is a drug that combines ectacapone and carbidopa-levodopa in one pill.
MAO-B inhibitors
Pitfalls Limitations And Alternative Interpretations
Given that the Swedish health care system typically offers everyone a life-long follow-up, it is unlikely that RA remains undiagnosed in RA-affected individuals or that PD remains undiagnosed in PD-affected individuals. It is impossible to fully exclude that the association we detected was caused by a confounder, however, we took great care to match PD cases and controls in order to make the groups as similar as possible. A potential confounder that we did not account for could be, for example, educational attainment. Shorter education is associated with increased prevalence of RA in Sweden . Since it has conversely been shown that men attaining higher IQ scores during military conscription in Sweden exhibit higher risk of developing PD , education level is potentially a confounder that could influence the risk for both diseases.
It is important to highlight that our working dataset was limited to only individuals who were first-degree relatives of a person diagnosed with diabetes mellitus or celiac disease. While this dataset was limited, it still allowed us to include 3.6 million individuals, or more than 20% of the entire population followed in the registry. We took precautions to ensure that PD cases and controls were also matched based on the exact type of a relative who had PD and RA. The matching was intended to prevent potential fictitious associations that could arise, due to unequal distribution of family-size affecting factors between PD case and control groups.
Potential New Treatment Strategies For Parkinson’s
This new finding provides additional knowledge and understanding of the disease processes that are present in PD and opens the door for using immunotherapies, drugs that suppress the abnormal immune response seen in autoimmune disorders. Additional research is needed to understand the molecular steps that occur in PD and the immune response, but researchers are hopeful that an immunotherapy strategy could help to prevent or lessen worsening symptoms in people with PD.1,2
Introduction: Parkinson’s Disease And Inflammation
Multiple studies have highlighted an association between sustained inflammation and a number of neurodegenerative diseases including Alzheimer’s disease , Parkinson’s disease , amyotropic lateral sclerosis , and frontal temporal dementia . The role of inflammation in the pathogenesis of these disorders, however, remains undetermined. Here, we focus on the inflammatory features in PD, the most common movement disorder, affecting more than 10 million people worldwide . PD patients manifest motor symptoms including bradykinesia, rest tremor, muscular rigidity, and postural and gait impairment, as well as non-motor symptoms . Non-motor symptoms include mood disorders, cognitive impairments, and autonomic dysfunction, such as orthostatic hypotension and constipation . While alleles of many genes are associated with the disorder, PD remains largely a sporadic disorder associated with older age and various genetic and environmental risk factors .
PD is diagnosed from motor symptoms , but non-motor symptoms are often manifest during a prolonged prodromal phase as much as 20 years prior to the onset of the motor features . These prodromal non-motor symptoms include constipation, rapid eye movement sleep behavior disorder, depression, anosmia, and excessive daytime sleepiness . The sensitivity and the specificity of these non-motor symptoms limits their utility in predicting the development of PD .
Investigating Parkinsons As An Autoimmune Disease
At the University of Montreal, Professor Louis-Eric Trudeau investigates the earliest potential causes of Parkinson’s disease, at the cellular level. His project is funded by the Saucier-van Berkom Parkinson Quebec Research Fund with contributions from Parkinson Newfoundland & Labrador, in the amount of $49,748.00 for 1 year. He is exploring the possibility that Parkinson’s is a form of autoimmune disease, caused when the immune system attacks the axon terminals in our brain cells. Those extremities release the chemical messengers that communicate with other cells, and damage to such terminals may disrupt the dopamine-producing brain cells that are key to Parkinson’s.
The death of the brain cells that produce dopamine, the chemical messenger that signals other cells involved in motor control, triggers the symptoms of Parkinson’s disease. But researchers still don’t know exactly what causes those dopamine-producing cells to die.
Trudeau, a neuroscientist, investigates the possibility that an autoimmune attack on those dopamine cells is the culprit.
Trudeau and his immunologist colleague, Michel Desjardins, are studying the role of the portion of cells called axon terminals. These terminals—the root-like extremities of cells—release the chemical messengers that send communication signals. Trudeau believes the death of these terminals, before the death of the dopamine cells themselves, is where the trouble starts.
Changes In T Cell Subpopulations And Cytokines
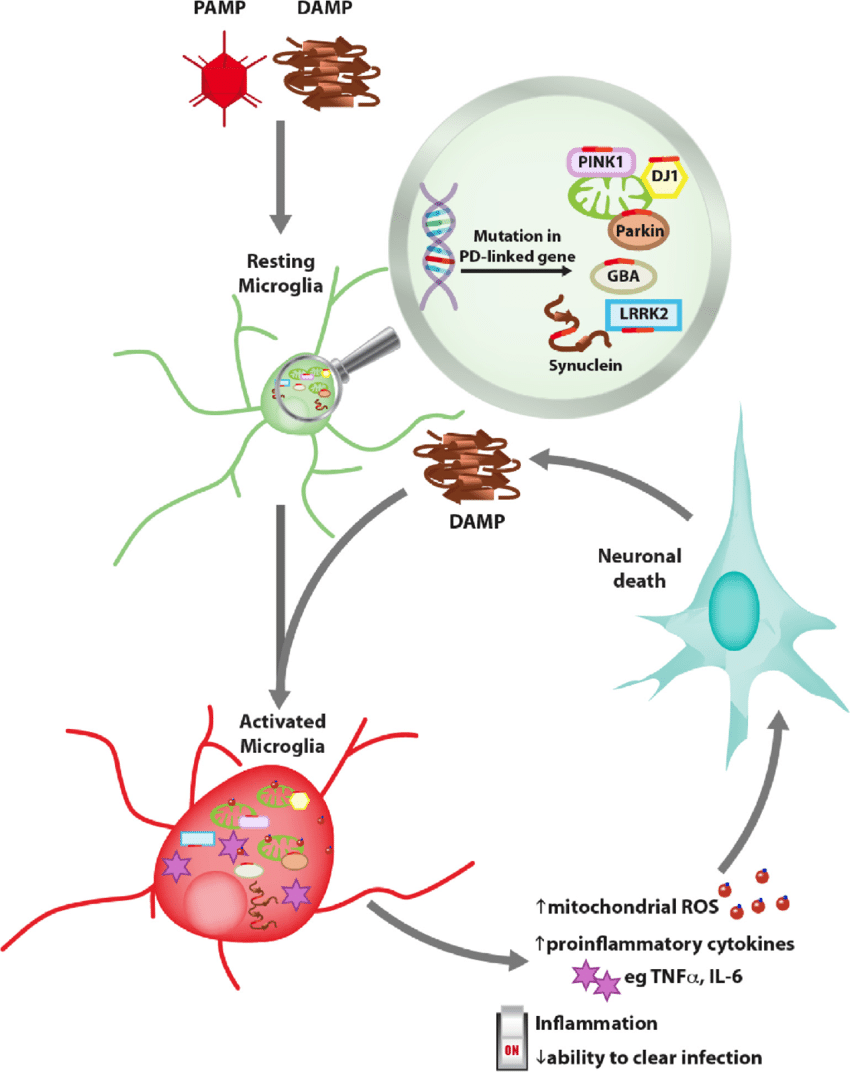
Consistent with the “systemic” view that PD involves multiple systems and tissues, several studies have shown general alterations in cytokines and immune cell populations.
Proinflammatory cytokines are elevated in the blood of PD patients, including increased levels of IL-2 ?6 ?8 , MCP-1 , MIP-1? , RANTES , TNF? , and IFN? . Increased levels of proinflammatory cytokines and chemokines are indicative of an immune system responding to tissue damage and/or foreign molecules. The levels of cytokines and chemokines correlate with the clinical stage of the disease, highlighting a role for peripheral inflammation in PD progression . Altered T cells populations can also contribute to the changes in circulating cytokines. Th1 and TH17+ CD4+ cells can contribute to the increased levels of IFN?, TNF?, and IL-17 .
Advances To Prevent The Symptoms Of Parkinsons
These findings suggest a possible treatment. In this case, immunotherapy could be used to increase the tolerance of the immune system to this protein. This could help improve or prevent the worsening of symptoms in patients with Parkinson’s. It could also provide a diagnostic test to identify the individuals most at risk. And those who are in the early stages of the disease.
This research, together with other work, such as that being carried out at the Karolinska Institute in Stockholm, which has found that the disease could originate in the bowels and spread to the brain through the vagus nerve, shows that progress is gradually being made in improving knowledge about a disease that affects more than six million people around the world.
Pathogenic Protein Function In Autoimmunity
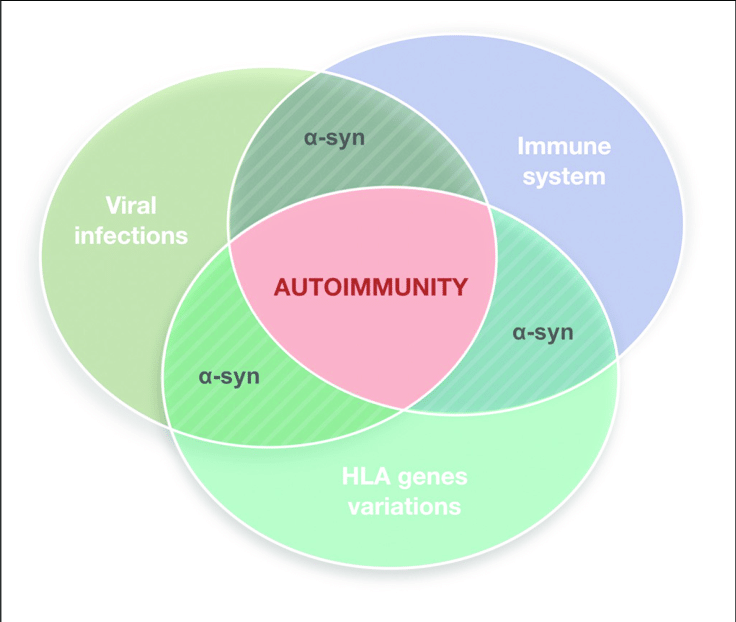
As discussed above, molecular mimicry and cross immunoreactions are two of the primary mechanisms through which autoimmunity is triggered. Molecular mimicry between herpes simplex virus 1 and human ?-syn was detected in PD patients in 2016. HSV1 infection could enhance the development of autoimmunity because autoreactive antibodies induced by HSV1 have the same response to the human ?-syn homologous peptide bound to the membrane of DNs and lead to DN destruction . These results also support the assumption that ?-syn participates in autoimmunity involved in the pathological progression of PD.
The Role Of Mhc Proteins In Pd Pathogenesis
T cells recognize a complex formed between MHC molecules and a peptide epitope. The epitope is presented to T cells as a result of a complex series of events termed antigen processing and presentation that involve the intracellular degradation of self and foreign proteins into peptides that are then loaded onto the antigen-binding groove of MHC molecules. In general, T cells recognize peptides derived from foreign molecules, but in disease some T cells also evade self-tolerance and can recognize self-peptides. MHC class I in general presents peptides derived from the same cell , while MHC class II typically present peptides derived from extracellular proteins .
Mature neurons are generally thought to lack MHC expression and so to not present antigens. However, SN and LC neurons recently have been shown to express MHC-I and co-localize with CD8+ T cells in response to stimuli such as IFN? . Catecholamine neurons are particularly sensitive to inflammation in comparison to other neuron types. A lower amount of IFN? is required by dopaminergic neurons to express MHC-I in comparison to other neuronal subtypes. The MHC-I presented by dopaminergic neurons is functional and can result in cytotoxicity in the presence of matching antigen and CD8+ T cells . Dopaminergic neuron sensitivity to inflammation and corresponding MHC-I upregulation could render them susceptible to infiltrating T cells versus neighboring, more resistant GABAergic neurons.
