Does The Brain’s Ability To Compensate For The Shortage Of Dopamine Affect Parkinson’s Disease Progression
Study Rationale:Parkinson’s disease is a progressive disorder, but it is unclear what causes the gradual worsening of the symptoms over the years. It is also unknown why in some cases the disease progresses very rapidly while in others it has a mild course. Here we aim to investigate which brain mechanisms are involved in the gradual worsening of motor symptoms in PD. More specifically, we aim to disentangle the contribution of two potential brain mechanisms: the progressive loss of dopamine, which causes dysfunction of the basal ganglia , and the inability of other brain regions not affected by Parkinson’s to compensate for basal ganglia dysfunction.
Hypothesis:We hypothesize that the speed of Parkinson’s disease progression depends on the brain’s ability to compensate for the shortage of dopamine.
Impact on Diagnosis/Treatment of Parkinson’s disease:This study will demonstrate how compensatory mechanisms, such as neuroplasticity, contribute to worsening of symptoms in Parkinson’s disease. This may help us predict an individual course of PD.
Next Steps for Development:If we confirm our hypothesis that the brain’s ability to compensate plays a larger role in shaping disease progression than does the dysfunction of the basal ganglia, then this may cause a paradigm shift in research, re-orienting therapeutic clinical trials from neuroprotection to neuroplasticity.
Analysis Of The Relationship Between Type Ii Diabetes Mellitus And Parkinsons Disease: A Systematic Review
Fauze Camargo Maluf
1Medical Student of Centro Universitario Saude ABC, Centro Universitario Saude ABC, FMABC, Santo Andre 09060-870, Brazil
2Department of Pharmacology, Centro Universitario Saude ABC, FMABC, Santo Andre 09060-870, Brazil
3Department of Neurosciences, Centro Universitario Saude ABC, FMABC, Santo Andre 09060-870, Brazil
Abstract
1. Introduction
The prevalence of type 2 diabetes mellitus is 370 million people in the world. The T2DM is most frequent in adulthood; however, in the last years, the prevalence of T2DM is increasing in adolescents and children . T2DM is a chronic metabolic disease characterized by long-term insulin resistance and a decrease of ?-cell function and population. These factors impair insulin release and consequently cause hyperglycemia . However, genetic and environmental factors are responsible for 20% of ?-cell failure in the diabetic population .
One of the consequences of chronic diabetes is the production of toxic aggregates of the islet amyloid polypeptide . The IAPP might contribute to ?-cell dysfunction .
Parkinson’s disease affects about 1% of people over 65 and up to 4-5% of people over 85, and thus it represents the second most common neurodegenerative disorder .
The diagnosis of PD is still based on the presence of symptoms and clinical signs such as typical asymmetric manifestation, the most common finding being tremor at rest in the upper limbs associated with bradykinesia, rigidity, and gait difficulty .
3. Results
Downstream Effects Of Dopal Accumulation: Oxidative Stress Mitochondrial Dysfunction And Cell Death
A further analogy with DA is that also DOPAL quinones could covalently modify mitochondrial protein, possibly affecting mitochondrial physiology . In the work by Kristal et al., isolated mitochondria from mouse liver were exposed to DOPAL resulting in an increased opening of the mitochondrial permeability transition pore at concentrations close to physiological ones . Later studies reported that DA oxidation to quinones induced mitochondria swelling and reduced respiratory activity, suggesting the induction of the mPTP opening . An analogous effect was ascribed to DAQs derived from enzymatic oxidation of DA, specifically addressing the modulation of mPTP opening to DAQs . As a consequence, both DA and DOPAL-derived quinones could be responsible for the activation of the apoptotic pathway. On the other hand, DOPAL-induced decreased cell viability was assessed by measuring Lactate Dehydrogenase release in the extra-cellular space, which is an accepted indication of necrosis .
There Is An Inverse Relationship Between Disease Severity And Gaba Levels In The Motor Cortex
There are no previous reports of changes in GABA levels in the motor cortex of Parkinson’s disease patients. However, our finding that lower GABA levels were associated with higher disease severity is in line with previous work using other modalities: fMRI results showed increased motor cortex BOLD activity in Parkinson’s disease during thumb pressing movements, and this effect correlated with higher rigidity scores . The positive relationship between motor cortex activity and disease severity may reflect the same mechanism as the inverse relationship between motor cortex inhibition and disease severity found here: several studies in healthy subjects showed that reduced GABA levels were associated with increased task-related BOLD responses in the cortex with examples from the motor cortex , the anterior cingulate cortex , and visual cortex .
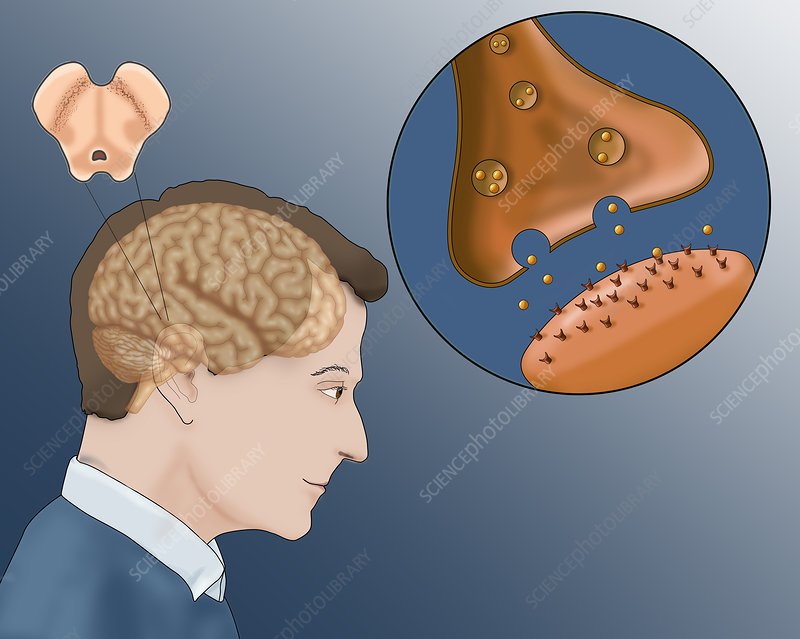
Parkinson’s Disease: Why Dopamine Replacement Therapy Has A Paradoxical Effect On Cognition
- Date:
- University of Montreal
- Summary:
- Dopamine replacement therapy, which is used to manage motor symptoms associated with Parkinson’s disease, can, at times, adversely affect cognition. Now researchers have identified the reasons why.
Dopamine replacement therapy, which is used to manage motor symptoms associated with Parkinson’s disease, can, at times, adversely affect cognition. Dr. Oury Monchi, Ph. D. in neuronal modeling and Head of the Neurophysiological and Neuroimaging Research theme at the Centre de recherche de l’Institut universitaire de gériatrie de Montréal , which is affiliated with the Université de Montréal, and Dr. Penny A. MacDonald, Neurologist and postdoctoral fellow in Dr. Monchi’s laboratory, have identified the reasons why within the framework of a clinical study recently published in Brain: A Journal of Neurology.
Until now, the effect of dopamine replacement therapy on cognition in individuals with Parkinson’s disease was controversial. The purpose of this study however, was to further investigate. This led to a series of laboratory tests and neuroimaging studies that allowed researchers to clearly define the distinct cognitive functions performed by the dorsal and ventral striatum, thereby shedding some light on the issue.
Summary of the Research
Parkinson’s disease
The authors are grateful for the support provided by the IUGM Foundation and the Canadian Institutes of Health Research.
Story Source:
Diabetes A Contemporary Risk For Parkinsons Disease: Epidemiological And Cellular Evidences
- 1Nutrition and Health Substantiation Group, Nutrition and Health Program, Health and Biosecurity, Commonwealth Scientific and Industrial Research Organisation , Adelaide, SA, Australia
- 2Adelaide Medical School, University of Adelaide, Adelaide, SA, Australia
- 3Cellular Neurobiology, Department of Medical Biology, Université du Québec, Trois-Rivières, QC, Canada
- 4Department of Biomedical Sciences, University of Cagliari, Cagliari, Italy
- 5National Institute for Neuroscience , University of Cagliari, Cagliari, Italy
- 6Department of Psychiatry and Neuroscience, Université Laval and CHU Research Center, Québec, QC, Canada
Dopamine Release And Neuronal Calcium Sensor 1: Possible Implications In Parkinson Disease
Among the Ca2+-binding proteins, the components of the subfamily of Neuronal Ca2+ Sensors are particularly abundant in neurons and photoreceptors and deserve special attention since their properties distinguish them from CaM or CB-28K, CR and PV and allow them to play non-reduntant roles. Differences in Ca2+ affinities, in cellular expression and distribution and in target proteins are at the basis of the specialization of NCS function . Neuronal Ca2+ Sensor-1 is the most ancient member of the family , and it is implicated in the regulation of cell-surface receptors and ion channels, and in neurotransmitter release, gene transcription, cell growth and survival .
NCS-1 has been linked to a large spectrum of diseases possibly because its differential interaction with partners. Changes in the abundance of NCS-1 result in altered relationship with target proteins and determine cell dysfunction. An up-regulation of NCS-1 mRNA was found in a variety of non-neurological and neurological diseases. NCS-1 has been proposed to be a biomarker in aggressive breast cancer . In the heart, altered Ca2+ signaling mediated by NCS-1 and inositol 1,4,5 trisphosphate receptor interaction was linked to cardiac arrhythmias . Schizophrenia, bipolar disorder and autism have been associated with upregulation or mutations in NCS-1 protein.
Microglial Activation And Systemic Chronic Inflammation Increase The Risk Of T2dm And Pd
Microglia are mononuclear, phagocytic immune cells in the central nervous system. They are normally involved in removal of damaged neurons, and they release neuroprotective factors to promote synaptic regeneration . Microglia can be activated towards either an anti-inflammatory or inflammatory phenotype. For example, microglia can be stimulated by lipopolysaccharide to enter an activated inflammatory state and express pro-inflammatory cytokines such as TNF?, interleukin 1? and IL6, triggering neuroinflammation . A study of 14 patients with PD found evidence of increased microglial activation on PET imaging . Microglial activation is a key contributor to neuroinflammation through the release of inflammatory cytokines , and patients with PD have been shown to have high concentrations of inflammatory mediators such as IL1?, IL6 and TNF? in the brain .
Parkinsons Disease Calcium And Selective Vulnerability Of Substantia Nigra Par Compacta
In summary, if by one side Ca2+ entry through Cav1.3 pore subunit is essential to sustain pacemaking activity of SNc DA neurons, by the other it exposes these neurons to metabolic burden and mitochondrial stress. Differently, DA neurons from the ventral tegumental area , which are also autonomous pacemakers, are significantly less vulnerable than SNc DA neurons from which they differ in respect with two main features: they have smaller Ca2+ currents and strong intrinsic Ca2+ buffering capacity due to higher calbindin levels .
The most convincing argument in favor of the “Ca2+ hypothesis” in PD onset is that epidemiologic studies on patients under clinical trial with L-type channel antagonists for the treatment of hypertension have shown a reduced risk of developing PD . The voltage gated L-type Ca2+ plasma membrane channels inhibitor isradipine has been demonstrated to be neuroprotective in a mouse model of PD and phase III of clinical trial is currently under evaluation to establish whether treatment with isradipine is able to slow the progression of PD in humans .
Despite general consensus agrees with the fact that the anatomical, physiological, and biochemical phenotype of the SNc DA neurons predisposes them to mitochondrial dysfunction, the molecular bases of the subtype-selective neuronal vulnerability are still obscure and of big interest.
What Should I Do If Im Managing Type 2 Diabetes And Concerned About My Parkinsons Risk
The takeaway of the new analysis for people currently managing or caring for a person with diabetes is unclear. This specific research, for example, doesn’t illustrate how someone with diabetes may help lower their risk of Parkinson’s disease, says Dr. Cereda.
“Unfortunately, although there is some evidence that diabetes is a risk factor for developing Parkinson’s disease, there is no evidence that optimal diabetes control reduces the risk of Parkinson’s disease,” says Cereda.
Yet managing blood sugar is still essential for people with type 2 diabetes, because failing to do this increases the risk of a wide range of health problems including heart disease, stroke, and kidney failure, Cereda says. The study results suggest that we might one day add Parkinson’s disease to the long list of conditions that can be prevented at least in part by good diabetes management, Cereda adds.
Noyce agrees, emphasizing the importance of blood sugar management regardless of potential Parkinson’s risk. “There are many other negative health outcomes that are associated with type 2 diabetes, such as heart disease, stroke, nerve and kidney damage, and visual loss,” Noyce says. “These are all more common than Parkinson’s, and the risk of these things can be reduced with treatment of diabetes, modification of diet, exercise and self-care.”
Type 2 Diabetes Associated With Increased Risk Faster Progression Of Parkinson Disease
A study finds type 2 diabetes associated with an increased risk of developing Parkinson disease , as well as faster progression of motor symptoms in those with PD.
Patients with type 2 diabetes may be at greater risk for developing Parkinson disease , with T2D also associated with faster disease progression in those with PD, according to study findings published this week in Movement Disorders.
As 2 prevalent diseases within an aging population, prior research has highlighted the biologic similarities between T2D and PD.
“Both are characterized by aberrant protein accumulation, lysosomal and mitochondrial dysfunction, and chronic systemic inflammation,” explain the study authors. “Insulin resistance is a hallmark of T2D and may be an important contributing factor to PD, too.”
In addition to studies on the relationship between the 2 diseases, prior systematic reviews and meta-analyses have investigated whether T2D may contribute to risk of developing PD. Although findings are conflicting, the researchers note that most of these studies recruited cohorts of patients with diabetes, as opposed to T2D, with this association also not explored via modern causal methods.
In the meta-analysis, pooled effect estimates showed that T2D was associated with an increased risk of PD , a causal relationship supported by MR .
Reference
Involvement Of Oxidative Stress/mitochondrial Dysfunction In T2dm And Pd Pathogenesis
Mitochondrial proteins, when dysfunctional, produce an increase in oxidative stress and cell death . MPTP exerts its Parkinson’s-like effects in rodent models by selectively inhibiting complex I, the first enzyme in the mitochondrial respiratory chain pathway, leading to neuronal death and neurodegeneration . The features of mitochondrial dysfunction may be shared in T2DM and PD . In PD, dysfunctional insulin signalling has been found to increase oxidative stress , while a recent study showed that chronic insulin resistance in diabetic db/ db mice can cause mitochondrial disruption and dopaminergic neuronal degeneration . Studies using rodent models show that IRS1 and IRS2 inhibit FOXO1 via the PI3K/Akt pathway , resulting in dysfunctional ATP generation and fatty acid oxidation, and the generation of ROS and oxidative stress. While the exact mechanism by which mitochondrial dysfunction and oxidative stress contribute towards PD remains uncertain, its role is likely to be important in PD pathogenesis and potentially relevant to the link with T2DM.
Mitochondrial Dysfunction: A Pivotal Pathological Mechanism Of Parkinsons Disease
Mitochondria are complex cytosolic organelles of eukaryotic cells whose primary function is the generation of cellular energy in the form of ATP by oxidative phosphorylation. Mammalian mitochondria contain between 2 and 10 mitochondrial DNA molecules encoding 22 transfer RNAs, two ribosomal RNAs, and 13 polypeptides, each of which is part of the respiratory chain and the oxidative phosphorylation system . The mitochondrial respiratory chain contains four protein complexes that form the site of oxidative phosphorylation. This site is responsible for NADH and FADH2 oxidation, co-occurring with the movement of protons from the matrix into the intermembrane space. This movement produces an electrochemical gradient denoted as mitochondrial membrane potential . This gradient stimulates the ATP synthase to reduce molecular oxygen and synthesize ATP. This step is fundamental in aerobic metabolism and constitutes the primary provider of ATP at the final stage of cellular respiration . Nevertheless, the biological function of mitochondria goes far beyond energy production and includes the metabolism of lipids and amino acids and the support of intermediate metabolic pathways, such as the Krebs cycle.
Dopaminergic Input And Organizational Features Of The Dorsal And Lateral Striatum
As reviewed above, it is generally accepted that dysfunction in PD stems from the degeneration of SNc neurons , which leads to motor dysfunction and the loss of VTA neurons , which leads to behavioral dysregulation, including demotivation, anhedonia, and depression within PD . While both pathways have been studied extensively across an array of conditions and pathologies, the modulatory mechanisms of the nigrostriatal pathway neurons have been fairly well described while the varied mechanisms and roles of VTA efferents continue to be elucidated. Within the nigrostriatal pathway, GABAergic medium spiny neurons of the dorsal/lateral striatum receive excitatory glutamatergic signals that can be modulated via dopaminergic inputs originating from the SNc. MSNs are moderately sized cells with large, multi-structured dendritic arbors that constitute a staggering 95% of all postsynaptic nigrostriatal neurons . Local circuit interneurons of the dorsal striatum are also actively involved in regulating MSN activity and can be subdivided into cholinergic interneurons and aspiny GABAergic interneurons known as low-threshold, fast-spiking neurons . Striatal cholinergic and MSNs express several neurotransmitter receptors including the ?-aminobutyric acid , glutamate, DA, adenosine, serotonin, opioids, and substance P receptors .
Study Examines Connection Between Diabetes Medication And Parkinsons Disease
It was first suggested in the 1960’s that people with type-2 diabetes are at increased risk for developing Parkinson’s disease – and when they do develop PD, its progression is faster and often more severe. This may be due, in part, to an apparent relationship in the brain between dopamine, insulin resistance, and glucose control. Insulin is not only made in the pancreas, it’s also present in the brain – where it has been shown to impact dopamine levels.
Parkinson’s is generally believed by scientists to be caused by the loss of dopamine-producing neurons. Parkinson’s symptoms, such as slowness, rigidity, and tremor, typically develop after approximately 40-80% of these dopamine-producing neurons die.
Why does this matter? Currently, more than 30 million people in the United States have type-2 diabetes, and that number is growing. The lifetime is also on the rise. In light of these trends, it would be valuable to know whether any specific type-2 diabetes medications might be associated with an increased or decreased risk for developing PD.
1) Thiazolidinediones , like pioglitazone or rosiglitazone , which specifically target insulin resistance
2) Drugs, like albiglutide or dulaglutide , that mimick glucagon-like peptide-1 a hormone that promotes insulin secretion, and
3) Dipeptidyl peptidase 4 inhibitors, which increase GLP-1 levels, and lead to insulin secretion and lowering of blood sugar levels
Results
Learn More
Earlier Research On The Link Between Type 2 Diabetes And Parkinsons Disease
Some previous research has linked certain medications for type 2 diabetes to a lower risk of the development or progression of Parkinson’s disease.
A found Parkinson’s disease symptoms improved in participants who took exenatide, a diabetes drug in a family of medicines known as GLP1 agonists, and worsened when subjects took a placebo. Another , found that individuals with type 2 diabetes who took GLP1 agonists or another type of diabetes drugs known as DPP4 inhibitors had a lower risk of developing Parkinson’s disease.
Slightly elevated blood sugar or variations in blood sugar may contribute to the risk of Parkinson’s disease even in people without diabetes, according to a .
Age is the biggest risk factor for Parkinson’s disease, though, and genetics also account for up to 20 percent of the risk, Foltynie says.
RELATED: How to Keep Your Brain Healthy: A Conversation With Sanjay Gupta, MD
Links Between Insulin Resistance Metabolic Syndrome And Parkinsons Disease
Insulin resistance and metabolic syndrome are very common in the United States, but are also noted to have increased incidence in patients with Parkinson’s disease . Insulin resistance may precede the development of diabetes by many years, but with treatment, diabetes can be avoided.
Growing evidence now shows an association of insulin resistance and metabolic syndrome with worse symptoms and progression of Parkinson’s disease . Lima et al. have shown diabetes incidence in PD is associated with faster progression of both motor and cognitive symptoms.
Anatomy Morphology And Functional Organization Of The Midbrain Da System
The complexity of the dopaminergic system seems to coincide with evolutionary development given that the number, size, and distribution, as well as receptor subtypes of dopaminergic neurons in the brain, increases alongside phylogenetic complexity . For example, dopaminergic terminal fields arising from midbrain clusters are more prominent and less segregated in the neocortex of primates than in rodents .
Dopaminergic neurons in the midbrain are mainly located in the SNc and VTA, although some smaller clusters have been found elsewhere, for instance, the dorsal and median raphe nuclei . In a classic article by Dahlstroem and Fuxe , SNc and VTA DA neurons were characterized based on their organization and projection patterns, which, in rat, can be found discrete clusters . SNc neurons innervate the dorsal and lateral striatum, thus forming a nigrostriatal pathway , and are necessary for the initiation and control of motor movements. Accordingly, the degeneration of this pathway is considered to be responsible for much of the motor dysfunction associated with PD. The VTA innervates the ventral striatum, nucleus accumbens, and limbic and cortical areas, and this way forms the mesolimbic and mesocortical pathways .
How To Determine The Dopamine Level In The Brain Of Parkinsons Patients
There is no rigorous method that could directly access to Dopamine and monitor its changes in the brain. The currently used methods rely on brain imaging techniques which do not reliably detect and measure Dopamine changes in the human brain.
Recently, neuroscientists at the Massachusetts Institute of Technology, Cambridge have developed a method to measure Dopamine in the brain for a long period of time, more than a year. They have designed a sensor which they believe could be used to monitor the changes in Dopamine levels in brain areas where Dopamine is highly concentrated. The sensor is so small that it can be implanted in different parts of the brain. The sensor has been successfully tested on animals and hopefully will be available for human trials in the near future.
Gabaergic Changes In The Thalamocortical Circuit In Parkinson’s Disease
Radboud University Medical Centre, Donders Institute for Brain, Cognition and Behaviour, Department of Neurology, Nijmegen, The Netherlands
Medical University of Vienna, Department of Neurology, Vienna, Austria
Radboud University Nijmegen, Donders Institute for Brain, Cognition and Behaviour, Centre for Cognitive Neuroimaging, Nijmegen, The Netherlands
Radboud University Medical Centre, Donders Institute for Brain, Cognition and Behaviour, Department of Neurology, Nijmegen, The Netherlands
Correspondence
Rick C. Helmich, Radboud University Nijmegen, Donders Institute for Brain, Cognition and Behavior, Centre for Cognitive Neuroimaging, 6500 HB Nijmegen, The Netherlands.
Radboud University Medical Centre, Donders Institute for Brain, Cognition and Behaviour, Department of Neurology, Nijmegen, The Netherlands
Medical University of Vienna, Department of Neurology, Vienna, Austria
Radboud University Nijmegen, Donders Institute for Brain, Cognition and Behaviour, Centre for Cognitive Neuroimaging, Nijmegen, The Netherlands
Radboud University Medical Centre, Donders Institute for Brain, Cognition and Behaviour, Department of Neurology, Nijmegen, The Netherlands
Correspondence
Rick C. Helmich, Radboud University Nijmegen, Donders Institute for Brain, Cognition and Behavior, Centre for Cognitive Neuroimaging, 6500 HB Nijmegen, The Netherlands.
The Association Between Type 2 Diabetes Mellitus And Parkinsons Disease

Article type: Review Article
Affiliations: Barts and The London School of Medicine, Queen Mary University of London, London, UK | Reta Lila Weston Institute of Neurological Studies, UCL Queen Square Institute of Neurology, London, UK | Department of Clinical and Movement Neurosciences, University College London Institute of Neurology, London, UK | Preventive Neurology Unit, Wolfson Institute of Preventive Medicine, Queen Mary University of London, London, UK
Correspondence: Correspondence to: Alastair J. Noyce, MRCP, PhD, Preventive Neurology Unit, Wolfson Institute of Preventive Medicine, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Tel.: +44 207 882 5841; E-mail: .
Keywords: Parkinson’s disease, type 2 diabetes mellitus, epidemiology, therapeutics, mechanisms
DOI: 10.3233/JPD-191900
Journal: Journal of Parkinson’s Disease, vol. 10, no. 3, pp. 775-789, 2020
Abstract
Insulin Dysregulation May Be Involved In Pathophysiology Of Pd And T2dm
Insulin receptors are expressed in the basal ganglia and in the substantia nigra , which are the areas of the brain most affected in patients with PD. Studies using rodent models have shown that insulin resistance may cause reduced expression of surface dopamine transporters in the striatum , reduced dopamine turnover , and reduced insulin-dependent dopamine release in the striatum . 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine is a toxin that induces parkinsonism by producing oxidative stress in dopaminergic neurons, resulting in mitochondrial dysfunction and cell death, and MPTP treated rodents are one of the most commonly used animal models for PD . MPTP-treated mice have been observed to have simultaneous increases in pancreatic and midbrain expression of pro-inflammatory cytokines and ?-synuclein, hinting at potential organ-specific links between PD and T2DM .
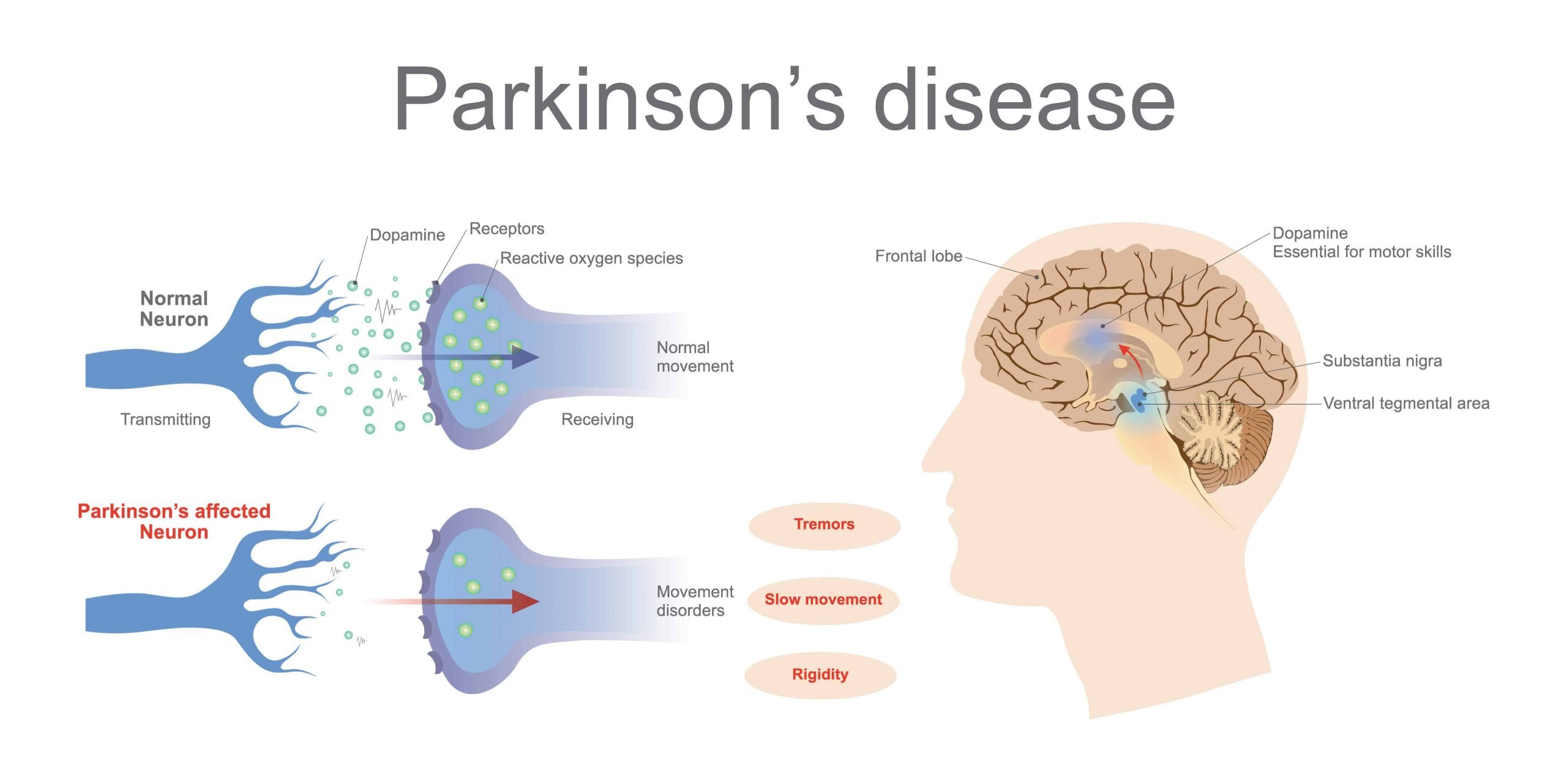
Parkinsons Disease And The Vulnerability Of The Nigrostriatal Pathway
PD is the prototype of movement disorders. In fact, the most prominent feature of PD is the presence of motor symptoms expressed in a majority of patients as a classical triad of resting tremor, bradykinesia, and rigidity . Non-dopaminergic motor features may also arise and result in falls, freezing of gait, speech impairment and difficulty in swallowing. Accompanying motor symptoms are equally disabling non-motor manifestations, such as psychiatric disturbances, dementia and autonomic failure .
The neuropathological hallmark of PD is the progressive and relentless degeneration of dopaminergic neurons located in the substantia nigra pars compacta. Idiopathic PD still composes approximately 90–95% of diagnoses that are not always faithfully represented by genetic forms in terms of symptomatology and pathophysiology .
Despite their apparent disparity, multiple mechanisms converge to cause the degeneration of nigrostriatal neurons and possibly contribute to oxidative stress to which these neurons may be more vulnerable . This concept dates back to the 1980s , but its popularity has waxed and waned at the rhythm of discoveries; nevertheless, the idea that metabolic oxidative insults may underlie the specific vulnerability of nigrostriatal neurons has lately gained momentum .
6. Last, they are endowed with a limited calcium-buffering capacity and scanty endogenous antioxidative defenses , consequent of their low expression of calbindin and glutathione , respectively.
Common Pathogenic Mechanisms Of Systemic And Brain Insulin Resistance
As epidemiological evidence for a link between PD and T2DM accumulates, parallel experimental evidence indicates potential overlap in disease mechanisms and pathways. Systemic insulin resistance has long been an established key feature of T2DM. Recently, studies have found that insulin resistance is present in the brain in neurodegenerative diseases such as Alzheimer’s disease and other dementias , and PD . Both systemic and local insulin resistance may drive pathology in the brain. Systemic insulin resistance may do so through hyperglycaemia and its consequences , microvascular disease, chronic inflammation, and dysfunction of the blood brain barrier, which may be compounded by associated comorbidities such as hypertension, dyslipidaemia and renal impairment . Local brain insulin resistance may act via protein deposition and aggregation, and failure of clearance mechanisms, independent of systemic insulin resistance .
Metformin Linked To Increased Risk Of Dementia And Parkinsons Disease
Study finds connection between duration of therapy in senior patients and development of neurodegenerative disease.
In 2011, the Journal of Alzheimer’s Disease published the findings of a large Taiwanese study showing a protective effect against development of dementia in diabetes patients who were given oral antidiabetic agents. The cohort of over 100,000 subjects included patients over 50 with type 2 diabetes, who were free of dementia at initiation, and received either or both metformin and a sulfonylurea. The results suggested that while T2D carries a two-fold increase in the risk of dementia, use of metformin, sulfonylureas, or both can reduce the risk by up to 35% over eight years. Medscape recently reported that at AD/PD 2017 , a group of Taiwanese neurologists presented the results of their own study looking at possible risk increases for Alzheimer’s and Parkinson’s in people with type 2 diabetes, citing uncertainty about the effects of metformin on the risk of developing neurodegenerative diseases.
Practice Pearls:
- Type 2 diabetes has long been understood to carry an increased risk of developing neurodegenerative diseases.
- Until recently, metformin and sulfonylurea use in T2D has been associated with a decrease in risk for Parkinson’s and Alzheimer’s diseases.
- A recent study suggests metformin use can increase the risk of Parkinson’s and Alzheimer’s, but there are also concerns over the validity of the findings due to study design flaws.
References:
How Type 2 Diabetes May Contribute To The Risk Of Parkinsons Disease
Although the analysis wasn’t designed to determine how type 2 diabetes might cause Parkinson’s disease to develop or progress, it’s possible that systemic inflammation present with type 2 diabetes may contribute to Parkinson’s disease, says Noyce.
Vascular disease that develops with type 2 diabetes may also lead to impaired blood flow to the brain that hastens the development of Parkinson’s disease, hypothesizes Emanuele Cereda, MD, PhD, of the clinical nutrition and dietetics unit at Fondazione IRCCS Policlinico San Matteo in Pavia, Italy, who was not involved in the current study.
Another possibility is that the same processes that cause diabetes also cause the nerve cell degeneration present in Parkinson’s disease, says Tom Foltynie, PhD, a professor of neurology at University College London in the United Kingdom.
In particular, insulin resistance, the body’s inability to respond normally to the hormone insulin, may be involved in both type 2 diabetes and Parkinson’s disease, says Dr. Foltynie, who also was not involved in the current research.
RELATED: Why Some Researchers Are Calling Alzheimer’s a ‘Type 3 Diabetes’
Aldehyde Dehydrogenases As Downstream Targets In Parkinsons Disease
In the last decades, several studies reported alterations in ALDHs expression and activity levels in PD patients’ nigral tissues, providing further support to the DOPAL paradigm for neurodegeneration. Initial evidence came from oligonucleotide in situ hybridization experiments on human post-mortem midbrain from PD patients with unreported aetiology. Aldh1a1 mRNA was found markedly reduced in TH-positive neurons in SNpc of parkinsonian brains compared to controls . A following genome-wide transcriptomic assay on PD patients confirmed similar down-regulation of Aldh1a1 mRNA in SNpc together with other 139 genes, revealing alterations in ubiquitin-proteasome, heat shock proteins, iron and oxidative stress regulated proteins, cell adhesion/cellular matrix and vesicles trafficking genes . Of note, neither study reported alterations in Aldh2 mRNA levels.
Strategies For The Treatment Of Parkinsons Disease: Beyond Dopamine
- 1Laboratorio de Neurobiología, Facultad de Ciencias de la Salud, Universidad San Sebastián, Concepción, Chile
- 2Department of Biological Sciences, University of Limerick, Limerick, Ireland
- 3Health Research Institute, University of Limerick, Limerick, Ireland
- 4Department of Psychology and Neuroscience, Center for Neuroscience, University of Colorado, Boulder, CO, United States
- 5Research & Development Service, Bay Pines VA Healthcare System, Bay Pines, FL, United States
Parkinson’s disease is the second-leading cause of dementia and is characterized by a progressive loss of dopaminergic neurons in the substantia nigra alongside the presence of intraneuronal ?-synuclein-positive inclusions. Therapies to date have been directed to the restoration of the dopaminergic system, and the prevention of dopaminergic neuronal cell death in the midbrain. This review discusses the physiological mechanisms involved in PD as well as new and prospective therapies for the disease. The current data suggest that prevention or early treatment of PD may be the most effective therapeutic strategy. New advances in the understanding of the underlying mechanisms of PD predict the development of more personalized and integral therapies in the years to come. Thus, the development of more reliable biomarkers at asymptomatic stages of the disease, and the use of genetic profiling of patients will surely permit a more effective treatment of PD.
