Treatments In Phase I Trials
As Parkinsons researchers work toward more precision medicines, they benefit from a wide range of therapeutic categories & tools to experiment with. Therapeutic categories refer to all the different categories of research focuses, like stem cells, antioxidants, and gene targeting, for example. With trials spanning more than 15 therapeutics categories in the Phase I pipeline, it has a broad range of focuses. Making discoveries in more therapeutic categories means we can one day provide access to personalized treatment solutions based on the specific causes and symptom presentation of each person living with Parkinsons.
There are 50 treatments in Phase I trials this year with about a 50/50 split between symptom-relieving products and disease-modifying treatments. Half of the treatments in Phase I are new discoveries as the result of pathfinding research. This is when researchers use insights from existing medications to seek out related products that warrant exploration.
A New Era For Parkinsons Disease Treatment
March 2, 2022 | By
A non-invasive ultrasound treatment for Parkinsons disease that was tested in a pivotal trial led by University of Maryland School of Medicine researchers is now broadly available at the University of Maryland Medical Center .
Howard Eisenberg, MD, Dheeraj Gandhi, MD, MBBS, Paul Fishman, MD, PhD, Bert W. OMalley, MD.
The device, called Exablate Neuro, was approved in November by the U.S. Food and Drug Administration to treat advanced Parkinsons disease on one side of the brain. The approval was based on findings from the UMSOM clinical trial and effectively expands access to focused ultrasound beyond clinical trial participation.
Rapid Reversal of Symptoms
Focused ultrasound is an incisionless procedure, performed without the need for anesthesia or an in-patient stay in the hospital. Patients, who are fully alert, lie in a magnetic resonance imaging scanner, wearing a transducer helmet. Ultrasonic energy is targeted through the skull to the globus pallidus, a structure deep in the brain that helps control regular voluntary movement. MRI images provide doctors with a real-time temperature map of the area being treated. During the procedure, the patient is awake and providing feedback, which allows doctors to monitor the immediate effects of the tissue ablation and make adjustments as needed.
Patient: Focused Ultrasound Changed My Life
A New Era for Parkinsons Disease Treatment
Amneal Tests A New Formulation
AmnealPharmaceuticals plans to report Phase III safety results for IPX-203, a reformulation of the common generic PD treatment combination of carbidopa and levodopa that could reduce symptom fluctuations. The company said the Phase III, open-label extension study will have results available by the end of the second quarter of 2022.
CD/LD can lead to troughs and spikes of plasma levels that generate side-effects like dyskinesia, Kordower explains. A new extended-release version of CD/LD could smooth out these drops, he notes.
If approved, IPX-203 will join several other marketed reformulations of CD/LD. Amneals own extended-release capsule Rytary, Schwarz Pharma s orally disintegrating tablet Parcopa, and AbbVie’s enteral suspension Duopa all have FDA approval in PD. A GlobalData consensus forecasts pegs peak IPX-203 sales at $127 million in 2028.
In a separate, placebo-controlled Phase III trial , IPX-203 resulted in 0.53 more hours of ON time than immediate-release CD/LD after seven weeks . Earlier, a six-week Phase II trial of IPX-203 reported no serious treatment-emergent adverse events among the 26 patients enrolled. Experts say the long-term safety data will be key in determining IPX-203s place among CD/LD formulations.
Don’t Miss: A Form Of Parkinson Disease
Treatments In Phase Iii Trials
What we see in Phase III trials this year is a total of 22 treatments from five different therapeutic categories. Three treatments in Phase III are disease-modifying treatments that have the potential to alter the progression of Parkinsons disease. One is widely used in traditional Chinese medicine, one is a repurposed Alzheimers medication, and the other is a repurposed diabetes medication.
There are also 19 studies in Phase III addressing symptoms of Parkinsons. While the community waits for gains to be made with disease-modifying treatments, improvements in symptom relief are critical for maintaining the quality of life. more personalized treatment plans If the symptom management products in Phase III safely and successfully complete their trials, the medical community will have more tools available to support Parkinsons symptom management.
More On Symptom Treatment And Disease

In 2022, 32 trials will be completed, including three stem cell trials. The decisions about which will move on to the next steps will be captured in the 2023 update. The current research landscape holds much promise. We are eagerly awaiting the results of the 32 trials being completed this year, what trials graduate to the next phases, and what new therapeutic categories will join the pipeline over the course of this year.
References
McFarthing, K., Rafaloff, G., Baptista, M., Mursaleen, L., Fuest, R., Wyse, R. K., & Stott, S. . Parkinsons Disease Drug Therapies in the Clinical Trial Pipeline: 2022 Update. Journal of Parkinsons disease, 12, 10731082.
Parkinson Canada’s mission is to transform the lives of people living with Parkinson’s across Canada. Articles like this represent our commitment to building awareness and providing resources and support for people living with Parkinson’s and their care network.
Find more posts about:
Read Also: What To Get Someone With Parkinson’s
Can You Stop A Parkinson’s Disease Tremor
“There is no cure for tremors caused by Parkinson’s disease. However, your doctor may prescribe some drugs to manage the symptoms. Some people might be able to relieve hand tremors by squeezing some objects like a stress ball. We are still waiting for a cure for Parkinson’s disease, but there is hope from clinical trials. Some patients are part of clinical trials that may offer promising drugs for Parkinson’s disease but must meet some requirements. Visit the Power website to find a clinical to learn more and find a clinical trial near you.” – Anonymous Online Contributor
Diet And Lifestyle Changes
Additional therapies for Parkinsons disease treatment include eating a healthy diet and engaging in regular exercise.
Some individuals may benefit from participating in physical and occupational therapy. These therapies often focus on balance, improving your gait, or tactics to allow you to complete your work.
Other alternative options center on promoting holistic well-being while living with Parkinsons disease. These are not shown to stop the diseases progression but can help you manage symptoms and stay hopeful:
You May Like: How Long Can One Live With Parkinson’s Disease
Brain Ultrasound Signals Linked To Motor Disability Gait
Enhanced ultrasound signals in the substantia nigra, the area of the brain impacted by Parkinsons disease, are associated with increased motor disability and gait disturbances, a study concluded. The researchers noted that these ultrasound signals, called hyperechogenicity, may be useful a biomarker that reflects disease severity. Our results may
A Study To Evaluate The Efficacy Of Prasinezumab In Participants With Early Parkinson’s Disease
Sorry, in progress, not accepting new patients
This multicenter, randomized, double-blind, placebo-controlled, Phase 2 study will evaluate the efficacy of intravenous prasinezumab versus placebo over 52 weeks in participants with early Parkinson’s Disease who are untreated or treated with monoamine oxidase B inhibitors since baseline. The study will consist of three parts: a 52-week, double-blind, placebo-controlled treatment period after which eligible participants will continue into an all-participants-on-treatment blinded dose extension for an additional 52 weeks . Participants who complete Part 2 will be offered participation in Part 3 open-label extension for an additional 260 weeks.
San Francisco, California
Read Also: Does Rls Lead To Parkinson’s
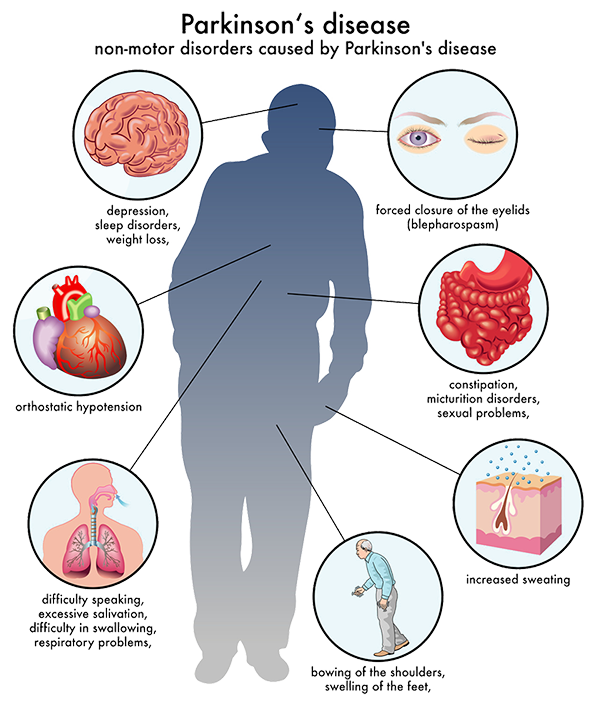
What Is Parkinson’s Disease
Parkinson’s Disease is a degenerative nervous system condition that affects one’s movement. Symptoms often start quite gradually, with minor issues such as small tremors within the extremities . Currently, there is no cure for Parkinson’s Disease but certain medications do have the capacity to help manage symptoms. Some doctors may also recommend surgery to address certain symptoms, which involves regulating certain areas of the brain.
What Is The Last Stage Of Parkinson’s Disease
“Stage 5 is the most severe stage of Parkinson’s disease. Once a patient reaches this stage, it means that they require life assistance most of the time. At this stage, a patient is unable to move because of physical impairment. There is no cure for Parkinson’s disease. However, there are clinical trials that have promising new drugs that can help patients who are part of the trials. If you know someone who could be part of Parkinson’s disease clinical trials, you may visit Power to learn more.” – Anonymous Online Contributor
Read Also: What Is The Difference Between Lewy Body Dementia And Parkinson’s
Protein Discovered In Parkinson’s Disease Could Lead To New Treatments
Currently, there are no disease modifying therapies for Parkinson’s disease that can change the progression of the disease. An international team of scientists led by faculty at the University of Colorado Anschutz Medical Campus is hoping to change that.
Today, they published new research in the journal Brain that takes scientists one step closer to understanding a key protein -synuclein , that they found links inflammation and Parkinson’s disease.
The protein Syn is predominantly expressed in neurons and is associated with neurodegenerative diseases like Parkinson’s disease and dementia with Lewy bodies. This new study identifies the novel mechanism that links interferon activation and Syn function in neurons as a potential trigger for developing Parkinson’s disease.
“It’s critical to understand further the triggers that contribute to the development of Parkinson’s disease and how inflammation may interact with proteins found in the disease. With this information, we could potentially provide new approaches for treatments by altering or interfering with these inflammatory pathways that may act as a trigger for the disease,” said David Beckham, MD, associate professor in the department of infectious disease at the University of Colorado School of Medicinelocated on the CU Anschutz Medical Campus.
Explore further
New Hope For Treatment Of Parkinsons Disease: Designer Neurons
The new research describes the implantation of induced pluripotent stem cells to replace dopamine-producing neurons destroyed by Parkinsons disease. Such cells not only survive the grafting procedure and manufacture dopamine, but send out their branching fibers through the neural tissue to make distant connections in the brain, just like their naturally-occurring counterparts. Credit: Shireen Dooling for the Biodesign Institute at Arizona State University
Neurodegenerative diseases cause neuronal damage and destruction, wreaking havoc on both mental and physical health. Parkinsons disease, which affects over 10 million people worldwide, is no exception. The most noticeable symptoms of Parkinsons disease arise after the illness damages a specific class of neuron located in the midbrain. The result is that dopamine, a key neurotransmitter produced by the affected neurons, is depleted in the brain.
In new research, Jeffrey Kordower and his colleagues describe a method for converting non-neuronal cells into functioning neurons able to take up residence in the brain, send out their fibrous branches across neural tissue, form synapses, dispense dopamine and restore capacities compromised by Parkinsons destruction of dopaminergic cells.
The work is supported through a grant from the Michael J. Fox Foundation.
New perspectives on Parkinsons disease
Neural alchemy
Read Also: What Benefits Can You Claim If You Have Parkinson’s
Cure Vcp Disease Patient Registry
If you have been diagnosed with a VCP gene mutation, please register yourself into our patient registry database. Collecting information on all of those with the disease will accelerate the path to drug development and potential therapies. This survey takes less than 20 minutes to complete. All information is secure and follows the strictest security protocols. You can choose to keep your information private from researchers, but we encourage you to enable researchers to see that information.
Our patient registry is hosted through CoRDS, a centralized international patient registry for all rare diseases. The goal of the CoRDS registry is to connect as many patients and researchers as possible to help advance treatments and cures for rare diseases. The CoRDS registry is free for patients to enroll and for researchers to access. CoRDS is part of Sanford Research, a not-for-profit research institution, based in Sioux Falls, SD.
What Causes Parkinson’s Disease
Parkinson’s Disease is caused by a loss of nerve cells in the brain. This loss of nerve cells within the brain results in a reduced amount of dopamine being created which acts as a messenger between the parts of your brain that control voluntary and involuntary movement. Therefore without that vital connection, your brain starts losing the ability to effectively control movement. Currently, it is unknown what causes the deterioration of nerve cells associated with Parkinson’s Disease . Currently, it is believed that both environmental factors, as well as genetic factors, may play a role in the loss of nerve cells.
Parkinson’s Disease is a lifelong condition that can greatly impair the ability of one’s daily functions. Traditional treatments only address the symptoms of the condition, but researchers are excited about the possibilities of certain gene therapies and stem cell therapy, which may have the ability to reverse damage and halt the progression of the disease.
Recommended Reading: What Is Pre Parkinson’s Disease
Rising Stars Live Online
Were pleased to host an exciting virtual event with some of our Rising Stars and, most notably, racers Tanner Foust and Loni Unser who will share their racing passion and experiences from the Pikes Peak International Hill Climb!
There is no charge for this virtual event, and participants can win great prizes from Xtreme Xperiences and Alpinestars!
Treatments In Phase Ii Trials
Another strategy in the therapeutic research space is drug repurposing. This is when an existing medication for one condition is repurposed to treat an entirely different condition. Working with repurposed medications comes with many advantages including understanding its general safety. Repurposing an existing medication, rather than starting from scratch, typically requires fewer tests for safety as the drug has already met these requirements. This can reduce costs and speed up the process through the clinical trial pipeline. It can also lead to faster approvals, getting much-needed treatments into the hands of people with Parkinsons as soon as possible. There are a total of 74 therapies in Phase II trials and 44% are repurposed medications.
One exciting takeaway from Phase II trials this year is the progress made with stem cell therapies. While there are nine stem cell therapies being explored in Phase I, two stem cell therapies graduated to Phase II trials this year! Moving into Phase II means these treatments are being administered to a larger group of people to monitor their effectiveness and further evaluate their safety.
Don’t Miss: How Is Parkinson’s Disease Causes
How Do Mesenchymal Stem Cells Work In The Body
Mesenchymal stem cells utilize their self-renewal, immunomodulatory, anti-inflammatory, signaling, and differentiation properties to influence positive change within the body. Mesenchymal stem cells also have the capacity to self-renew by dividing and developing into multiple specialized cell types present in a specific tissue or organ. Mesenchymal stem cells are adult stem cells, meaning they present no ethical concerns, MSCs are not sourced from embryonic material.
Parkinson Progression Marker Initiative Online
open to eligible people ages 18 years and up
Parkinson Progression Marker Initiative Online is an observational study collecting participant reported information from people with and without Parkinson’s disease , for the goal of better understanding risk and predictive factors for PD. PPMI Online is part of the broader Parkinson Progression Marker Initiative aimed at identifying markers of disease progression for use in clinical trials of therapies to reduce progression of PD disability.
San Francisco, California
You May Like: Learn About Parkinson’s Disease
What Are Mesenchymal Stem Cells
Stem cells are the body’s raw materials â cells from which all other cells with specialized functions are created. Mesenchymal stem cells are adult stem cells that have self-renewal, immunomodulatory, anti-inflammatory, signaling, and differentiation properties. Mesenchymal stem cells , self renewal capacity is characterized by their ability to divide and develop into multiple specialized cell types present in a specific tissue or organ.
Mesenchymal stem cells can be sourced from a variety of tissue including adipose tissue , bone marrow, umbilical cord tissue, blood, liver, dental pulp, and skin.
MSCs are widely used in the treatment of various diseases due to their self-renewable, differentiation, anti-inflammatory, and immunomodulatory properties. In-vitro and in-vivo studies have supported the understanding mechanisms, safety, and efficacy of MSC therapy in clinical applications.
Component #2 A Neuroprotective Agent
Once a drug or a treatment has been determined to slow down the progression of Parkinsons, it will be necessary to protect the remaining cells and provide a nurturing environment for the third part of the cure .
Neuroprotection is the area of research that has had the most attention over the years. Drug companies have employed vast resources in this area in the hope of discovering a treatment which will work across conditions , and thus provide them with tremendous profits. Unfortunately, conditions of the brain have proven to be a lot more complicated than first perceived and cross-condition therapies seem unlikely as we move towards greater stratification and personalisation of disease and treatment, respectively.
But there has been the hint of a potential neuroprotective effect in one class of drugs for Parkinsons: GLP-1R agonists.
Neuroprotective approach: GLP-1R agonists
Exenatide is a glucagon like peptide-1 receptor agonist. This is a class of drug that has traditionally been used for treating diabetes, but has recently been repurposed for Parkinsons.
After multiple studies suggested neuroprotective properties in models of Parkinsons, a clinical trial program was intiated, and in 2017, a Phase II Exenatide trial reported the stablisation of Parkinsons motor features over the course of the 48 week trial .
Reduction in motor scores in Exenatide group. Source: Lancet
In late 2019, we saw the initiation of a Phase III clinical trial for .
Also Check: Does Richard Blumenthal Have Parkinson’s
