Have You Come Across Advice That Those With Parkinsons Should Avoid Too Much Refined Sugar Or Perhaps Youre Aware Of New Research Linking Diabetes And The Condition We Go Behind The Headlines To Find Out What Is Actually Going On

Over the last decade, a war on sugar has started. In March 2016 the government announced that a tax on sugary soft drinks would be introduced in the UK from 2018. At the same time, we’ve seen a gradual increase in messaging that healthy eating, particularly limiting your intake of sugar, is essential for a healthy body.
The main aim of this war is to curb a growing obesity pandemic. And while it’s too early to draw any conclusions about these initiatives on the patterns of obesity in the UK, studies have suggested that simply reducing the sugar content of sweetened beverages by 40% over five years could result in roughly half a million fewer obese adults. As our collective national waistline shrinks, the hope is we will also see a reduction in a range of health conditions from heart problems to cancer, diabetes to osteoarthritis.
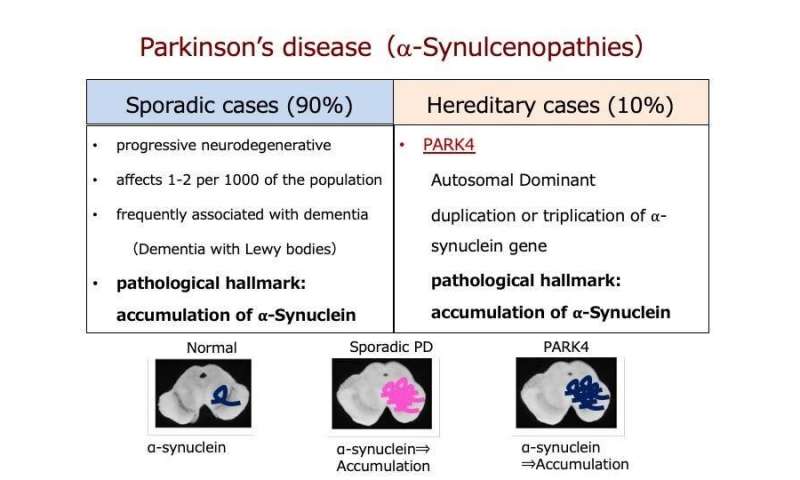
Analysis Of The Relationship Between Type Ii Diabetes Mellitus And Parkinsons Disease: A Systematic Review
Fauze Camargo Maluf
1Medical Student of Centro Universitario Saude ABC, Centro Universitario Saude ABC, FMABC, Santo Andre 09060-870, Brazil
2Department of Pharmacology, Centro Universitario Saude ABC, FMABC, Santo Andre 09060-870, Brazil
3Department of Neurosciences, Centro Universitario Saude ABC, FMABC, Santo Andre 09060-870, Brazil
Abstract
1. Introduction
The prevalence of type 2 diabetes mellitus is 370 million people in the world. The T2DM is most frequent in adulthood; however, in the last years, the prevalence of T2DM is increasing in adolescents and children . T2DM is a chronic metabolic disease characterized by long-term insulin resistance and a decrease of ?-cell function and population. These factors impair insulin release and consequently cause hyperglycemia . However, genetic and environmental factors are responsible for 20% of ?-cell failure in the diabetic population .
One of the consequences of chronic diabetes is the production of toxic aggregates of the islet amyloid polypeptide . The IAPP might contribute to ?-cell dysfunction .
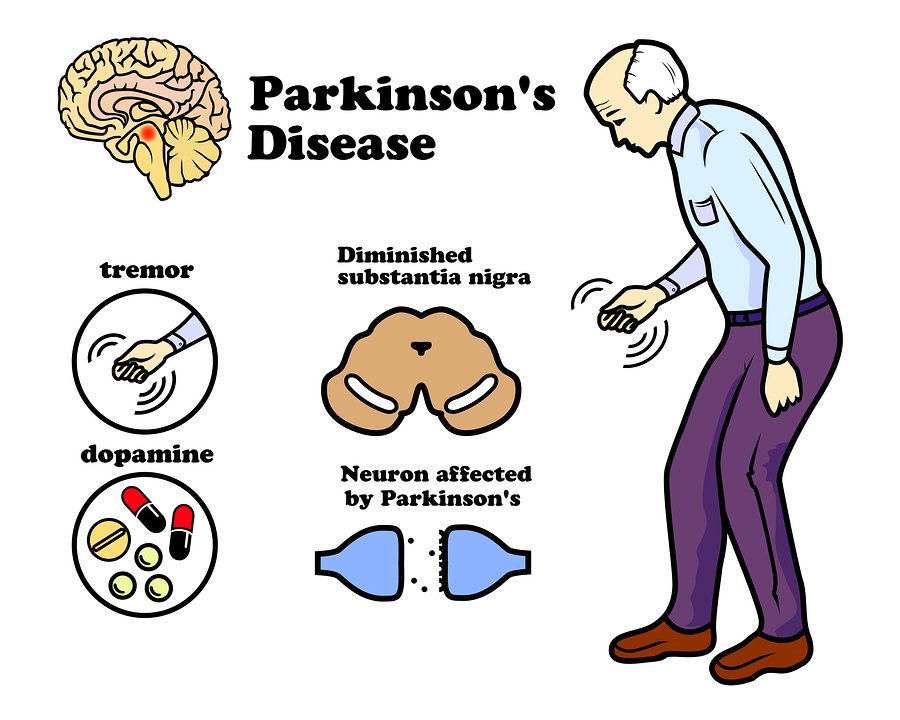
Parkinson’s disease affects about 1% of people over 65 and up to 4-5% of people over 85, and thus it represents the second most common neurodegenerative disorder .
The diagnosis of PD is still based on the presence of symptoms and clinical signs such as typical asymmetric manifestation, the most common finding being tremor at rest in the upper limbs associated with bradykinesia, rigidity, and gait difficulty .
3. Results
Diabetes A Contemporary Risk For Parkinsons Disease: Epidemiological And Cellular Evidences
- 1Nutrition and Health Substantiation Group, Nutrition and Health Program, Health and Biosecurity, Commonwealth Scientific and Industrial Research Organisation , Adelaide, SA, Australia
- 2Adelaide Medical School, University of Adelaide, Adelaide, SA, Australia
- 3Cellular Neurobiology, Department of Medical Biology, Université du Québec, Trois-Rivières, QC, Canada
- 4Department of Biomedical Sciences, University of Cagliari, Cagliari, Italy
- 5National Institute for Neuroscience , University of Cagliari, Cagliari, Italy
- 6Department of Psychiatry and Neuroscience, Université Laval and CHU Research Center, Québec, QC, Canada
Microglial Activation And Systemic Chronic Inflammation Increase The Risk Of T2dm And Pd
Microglia are mononuclear, phagocytic immune cells in the central nervous system. They are normally involved in removal of damaged neurons, and they release neuroprotective factors to promote synaptic regeneration . Microglia can be activated towards either an anti-inflammatory or inflammatory phenotype. For example, microglia can be stimulated by lipopolysaccharide to enter an activated inflammatory state and express pro-inflammatory cytokines such as TNF?, interleukin 1? and IL6, triggering neuroinflammation . A study of 14 patients with PD found evidence of increased microglial activation on PET imaging . Microglial activation is a key contributor to neuroinflammation through the release of inflammatory cytokines , and patients with PD have been shown to have high concentrations of inflammatory mediators such as IL1?, IL6 and TNF? in the brain .
What Should I Do If Im Managing Type 2 Diabetes And Concerned About My Parkinsons Risk
The takeaway of the new analysis for people currently managing or caring for a person with diabetes is unclear. This specific research, for example, doesn’t illustrate how someone with diabetes may help lower their risk of Parkinson’s disease, says Dr. Cereda.
“Unfortunately, although there is some evidence that diabetes is a risk factor for developing Parkinson’s disease, there is no evidence that optimal diabetes control reduces the risk of Parkinson’s disease,” says Cereda.
Yet managing blood sugar is still essential for people with type 2 diabetes, because failing to do this increases the risk of a wide range of health problems including heart disease, stroke, and kidney failure, Cereda says. The study results suggest that we might one day add Parkinson’s disease to the long list of conditions that can be prevented at least in part by good diabetes management, Cereda adds.
Noyce agrees, emphasizing the importance of blood sugar management regardless of potential Parkinson’s risk. “There are many other negative health outcomes that are associated with type 2 diabetes, such as heart disease, stroke, nerve and kidney damage, and visual loss,” Noyce says. “These are all more common than Parkinson’s, and the risk of these things can be reduced with treatment of diabetes, modification of diet, exercise and self-care.”
Type 2 Diabetes Associated With Increased Risk Faster Progression Of Parkinson Disease
A study finds type 2 diabetes associated with an increased risk of developing Parkinson disease , as well as faster progression of motor symptoms in those with PD.
Patients with type 2 diabetes may be at greater risk for developing Parkinson disease , with T2D also associated with faster disease progression in those with PD, according to study findings published this week in Movement Disorders.
As 2 prevalent diseases within an aging population, prior research has highlighted the biologic similarities between T2D and PD.
“Both are characterized by aberrant protein accumulation, lysosomal and mitochondrial dysfunction, and chronic systemic inflammation,” explain the study authors. “Insulin resistance is a hallmark of T2D and may be an important contributing factor to PD, too.”
In addition to studies on the relationship between the 2 diseases, prior systematic reviews and meta-analyses have investigated whether T2D may contribute to risk of developing PD. Although findings are conflicting, the researchers note that most of these studies recruited cohorts of patients with diabetes, as opposed to T2D, with this association also not explored via modern causal methods.
In the meta-analysis, pooled effect estimates showed that T2D was associated with an increased risk of PD , a causal relationship supported by MR .
Reference
Involvement Of Oxidative Stress/mitochondrial Dysfunction In T2dm And Pd Pathogenesis
Mitochondrial proteins, when dysfunctional, produce an increase in oxidative stress and cell death . MPTP exerts its Parkinson’s-like effects in rodent models by selectively inhibiting complex I, the first enzyme in the mitochondrial respiratory chain pathway, leading to neuronal death and neurodegeneration . The features of mitochondrial dysfunction may be shared in T2DM and PD . In PD, dysfunctional insulin signalling has been found to increase oxidative stress , while a recent study showed that chronic insulin resistance in diabetic db/ db mice can cause mitochondrial disruption and dopaminergic neuronal degeneration . Studies using rodent models show that IRS1 and IRS2 inhibit FOXO1 via the PI3K/Akt pathway , resulting in dysfunctional ATP generation and fatty acid oxidation, and the generation of ROS and oxidative stress. While the exact mechanism by which mitochondrial dysfunction and oxidative stress contribute towards PD remains uncertain, its role is likely to be important in PD pathogenesis and potentially relevant to the link with T2DM.
Study Examines Connection Between Diabetes Medication And Parkinsons Disease
It was first suggested in the 1960’s that people with type-2 diabetes are at increased risk for developing Parkinson’s disease – and when they do develop PD, its progression is faster and often more severe. This may be due, in part, to an apparent relationship in the brain between dopamine, insulin resistance, and glucose control. Insulin is not only made in the pancreas, it’s also present in the brain – where it has been shown to impact dopamine levels.
Parkinson’s is generally believed by scientists to be caused by the loss of dopamine-producing neurons. Parkinson’s symptoms, such as slowness, rigidity, and tremor, typically develop after approximately 40-80% of these dopamine-producing neurons die.
Why does this matter? Currently, more than 30 million people in the United States have type-2 diabetes, and that number is growing. The lifetime risk of developing Parkinson’s is also on the rise. In light of these trends, it would be valuable to know whether any specific type-2 diabetes medications might be associated with an increased or decreased risk for developing PD.
1) Thiazolidinediones , like pioglitazone or rosiglitazone , which specifically target insulin resistance
2) Drugs, like albiglutide or dulaglutide , that mimick glucagon-like peptide-1 a hormone that promotes insulin secretion, and
3) Dipeptidyl peptidase 4 inhibitors, which increase GLP-1 levels, and lead to insulin secretion and lowering of blood sugar levels
Results
What Does This Mean?
Learn More
Earlier Research On The Link Between Type 2 Diabetes And Parkinsons Disease
Some previous research has linked certain medications for type 2 diabetes to a lower risk of the development or progression of Parkinson’s disease.
A found Parkinson’s disease symptoms improved in participants who took exenatide, a diabetes drug in a family of medicines known as GLP1 agonists, and worsened when subjects took a placebo. Another , found that individuals with type 2 diabetes who took GLP1 agonists or another type of diabetes drugs known as DPP4 inhibitors had a lower risk of developing Parkinson’s disease.
Slightly elevated blood sugar or variations in blood sugar may contribute to the risk of Parkinson’s disease even in people without diabetes, according to a .
Age is the biggest risk factor for Parkinson’s disease, though, and genetics also account for up to 20 percent of the risk, Foltynie says.
RELATED: How to Keep Your Brain Healthy: A Conversation With Sanjay Gupta, MD
Links Between Insulin Resistance Metabolic Syndrome And Parkinsons Disease
Insulin resistance and metabolic syndrome are very common in the United States, but are also noted to have increased incidence in patients with Parkinson’s disease . Insulin resistance may precede the development of diabetes by many years, but with treatment, diabetes can be avoided.
Growing evidence now shows an association of insulin resistance and metabolic syndrome with worse symptoms and progression of Parkinson’s disease . Lima et al. have shown diabetes incidence in PD is associated with faster progression of both motor and cognitive symptoms.
The Association Between Type 2 Diabetes Mellitus And Parkinsons Disease
Article type: Review Article
Affiliations: Barts and The London School of Medicine, Queen Mary University of London, London, UK | Reta Lila Weston Institute of Neurological Studies, UCL Queen Square Institute of Neurology, London, UK | Department of Clinical and Movement Neurosciences, University College London Institute of Neurology, London, UK | Preventive Neurology Unit, Wolfson Institute of Preventive Medicine, Queen Mary University of London, London, UK
Correspondence: Correspondence to: Alastair J. Noyce, MRCP, PhD, Preventive Neurology Unit, Wolfson Institute of Preventive Medicine, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Tel.: +44 207 882 5841; E-mail: .
Keywords: Parkinson’s disease, type 2 diabetes mellitus, epidemiology, therapeutics, mechanisms
DOI: 10.3233/JPD-191900
Journal: Journal of Parkinson’s Disease, vol. 10, no. 3, pp. 775-789, 2020
Abstract
Insulin Dysregulation May Be Involved In Pathophysiology Of Pd And T2dm
Insulin receptors are expressed in the basal ganglia and in the substantia nigra , which are the areas of the brain most affected in patients with PD. Studies using rodent models have shown that insulin resistance may cause reduced expression of surface dopamine transporters in the striatum , reduced dopamine turnover , and reduced insulin-dependent dopamine release in the striatum . 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine is a toxin that induces parkinsonism by producing oxidative stress in dopaminergic neurons, resulting in mitochondrial dysfunction and cell death, and MPTP treated rodents are one of the most commonly used animal models for PD . MPTP-treated mice have been observed to have simultaneous increases in pancreatic and midbrain expression of pro-inflammatory cytokines and ?-synuclein, hinting at potential organ-specific links between PD and T2DM .
Parkinsons Disease And The Vulnerability Of The Nigrostriatal Pathway

PD is the prototype of movement disorders. In fact, the most prominent feature of PD is the presence of motor symptoms expressed in a majority of patients as a classical triad of resting tremor, bradykinesia, and rigidity . Non-dopaminergic motor features may also arise and result in falls, freezing of gait, speech impairment and difficulty in swallowing. Accompanying motor symptoms are equally disabling non-motor manifestations, such as psychiatric disturbances, dementia and autonomic failure .
The neuropathological hallmark of PD is the progressive and relentless degeneration of dopaminergic neurons located in the substantia nigra pars compacta. Idiopathic PD still composes approximately 90–95% of diagnoses that are not always faithfully represented by genetic forms in terms of symptomatology and pathophysiology .
Despite their apparent disparity, multiple mechanisms converge to cause the degeneration of nigrostriatal neurons and possibly contribute to oxidative stress to which these neurons may be more vulnerable . This concept dates back to the 1980s , but its popularity has waxed and waned at the rhythm of discoveries; nevertheless, the idea that metabolic oxidative insults may underlie the specific vulnerability of nigrostriatal neurons has lately gained momentum .
6. Last, they are endowed with a limited calcium-buffering capacity and scanty endogenous antioxidative defenses , consequent of their low expression of calbindin and glutathione , respectively.
Common Pathogenic Mechanisms Of Systemic And Brain Insulin Resistance
As epidemiological evidence for a link between PD and T2DM accumulates, parallel experimental evidence indicates potential overlap in disease mechanisms and pathways. Systemic insulin resistance has long been an established key feature of T2DM. Recently, studies have found that insulin resistance is present in the brain in neurodegenerative diseases such as Alzheimer’s disease and other dementias , and PD . Both systemic and local insulin resistance may drive pathology in the brain. Systemic insulin resistance may do so through hyperglycaemia and its consequences , microvascular disease, chronic inflammation, and dysfunction of the blood brain barrier, which may be compounded by associated comorbidities such as hypertension, dyslipidaemia and renal impairment . Local brain insulin resistance may act via protein deposition and aggregation, and failure of clearance mechanisms, independent of systemic insulin resistance .
Metformin Linked To Increased Risk Of Dementia And Parkinsons Disease
Study finds connection between duration of therapy in senior patients and development of neurodegenerative disease.
In 2011, the Journal of Alzheimer’s Disease published the findings of a large Taiwanese study showing a protective effect against development of dementia in diabetes patients who were given oral antidiabetic agents. The cohort of over 100,000 subjects included patients over 50 with type 2 diabetes, who were free of dementia at initiation, and received either or both metformin and a sulfonylurea. The results suggested that while T2D carries a two-fold increase in the risk of dementia, use of metformin, sulfonylureas, or both can reduce the risk by up to 35% over eight years. Medscape recently reported that at AD/PD 2017 , a group of Taiwanese neurologists presented the results of their own study looking at possible risk increases for Alzheimer’s and Parkinson’s in people with type 2 diabetes, citing uncertainty about the effects of metformin on the risk of developing neurodegenerative diseases.
Practice Pearls:
- Type 2 diabetes has long been understood to carry an increased risk of developing neurodegenerative diseases.
- Until recently, metformin and sulfonylurea use in T2D has been associated with a decrease in risk for Parkinson’s and Alzheimer’s diseases.
- A recent study suggests metformin use can increase the risk of Parkinson’s and Alzheimer’s, but there are also concerns over the validity of the findings due to study design flaws.
References:
How Type 2 Diabetes May Contribute To The Risk Of Parkinsons Disease
Although the analysis wasn’t designed to determine how type 2 diabetes might cause Parkinson’s disease to develop or progress, it’s possible that systemic inflammation present with type 2 diabetes may contribute to Parkinson’s disease, says Noyce.
Vascular disease that develops with type 2 diabetes may also lead to impaired blood flow to the brain that hastens the development of Parkinson’s disease, hypothesizes Emanuele Cereda, MD, PhD, of the clinical nutrition and dietetics unit at Fondazione IRCCS Policlinico San Matteo in Pavia, Italy, who was not involved in the current study.
Another possibility is that the same processes that cause diabetes also cause the nerve cell degeneration present in Parkinson’s disease, says Tom Foltynie, PhD, a professor of neurology at University College London in the United Kingdom.
In particular, insulin resistance, the body’s inability to respond normally to the hormone insulin, may be involved in both type 2 diabetes and Parkinson’s disease, says Dr. Foltynie, who also was not involved in the current research.
RELATED: Why Some Researchers Are Calling Alzheimer’s a ‘Type 3 Diabetes’
Epidemiological Evidence: Parkinsons Disease In Diabetic Patients
The first indications of a possible association between DM and PD date back to almost 60 years ago. Studies conducted in parallel showed that DM exacerbates the progression of motor and cognitive deficits in PD , and that non-diabetic parkinsonian patients present impaired glucose tolerance and hyperglycemia . Since then, these findings have been reiterated in parkinsonian patients medicated with dopamine-replacing therapy , but also in drug-naïve parkinsonian patients who still displayed higher-than-normal levels of fasting blood glucose, within the prediabetic diagnostic range . In addition, dietary habits featuring an elevated intake of high glycemic indexed carbohydrates have more recently been associated with greater odds of developing PD .
Despite the recent medical advances in teasing out the cause-effect relationship linking DM and PD, it is still unclear which of the multifarious metabolic changes that occur in DM trigger the nigrostriatal degeneration underlying PD. The two predominant lines of thought grant importance to the dimensions of defective insulin signaling and oxidative stress.
Rerouting Mechanisms: Polyol Pathway And Macromolecules Glycation
Aside from ROS generated through mitochondrial failure, hyperglycemia may trigger the onset of oxidative stress via pathways that dwell upstream the glycolytic enzyme GAPDH .
As previously discussed, free glucose is present in the cytosol of neurons and its concentrations rise during hyperglycemia leading to glucotoxicity. Under normal circumstances, hexokinase is mandated to transform available glucose into glucose 6-phosphate. However, due to substrate-mediated inhibition, in hyperglycemic conditions, it can only funnel a fraction of cytosolic glucose toward the glycolytic pathway before becoming saturated. Excess glucose is therefore consumed by other pathways and is constantly replenished by high extracellular levels. The principal compensatory outlets are the polyol pathway and macromolecule glycation which are two key pathways through which glucotoxicity manifests .
AGEs may also occur extracellularly or being directly secreted from the cells in which they are generated to induce oxidative stress and inflammation in a variety of cells . AGEs trigger oxidative stress by binding their cognate membrane receptor, the RAGE and activating downstream mediators know to induce oxidative stress, such as the inducible nitric oxide synthase . Nevertheless, despite AGEs being described for their ability to promote oxidative stress in peripheral tissues , their role in oxidative stress in PD remains to be elucidated.
Type 2 Diabetes Linked To Increased Risk Of Parkinsons Disease
Genetic data suggests there may be a direct relationship between type 2 diabetes and a higher risk of the movement disorder, as well as its progression, but more studies are needed.
Everyday Health
People managing type 2 diabetes and their doctors may need to have Parkinson’s disease on their radar, as new research sheds light on the possible connection between these two chronic health conditions.
For the review and meta-analysis, which was , researchers examined data combined from nine previous studies that followed individuals with type 2 diabetes over time to see if they developed Parkinson’s disease. They found type 2 diabetes associated with a 21 percent increased risk of Parkinson’s and with faster symptom progression. Parkinson’s causes muscle stiffness, tremors, impaired balance, and slow movement, in addition to cognitive and sleep issues, according to the National Institute on Aging.
Yet study authors could not account for the severity of participants’ type 2 diabetes, and they couldn’t determine the effect of diabetes drugs or quality of blood sugar management on Parkinson’s disease risk — two major limitations.
The research doesn’t prove that diabetes causes Parkinson’s, though researchers speculated the disease may contribute to this risk. They conducted a separate analysis of studies that identified common genetic variations present with the two conditions and found that type 2 diabetes directly increased the odds of developing Parkinson’s by 8 percent.
Cellular Evidence: Neuronal Oxidative Stress In Hyperglycemia
The clinical significance of hyperglycemia emerged upon its formal identification as the prime culprit in comorbid diabetic complications Group, 1998; Gaede et al., 2008; Giacco and Brownlee, 2010). The most affected collateral targets in DM are the kidneys, the cardiovascular system and the nervous system . Despite their many functional and phenotypic differences, the cells of these systems share the distinct liability of taking up glucose in an extracellular concentration-dependent but insulin-independent manner . Indeed, mesangial and endothelial cells predominantly express the insulin-independent glucose transporter GLUT1 ; likewise, brain cells express the insulin-independent GLUTs, GLUT1 and GLUT3 with the latter being particularly abundant in neurons . Although it is clear that glucose concentrations rise in these cells under conditions of hyperglycemia, how exactly glucotoxicity-mediated cellular damage occurs is not as forthright. The current consensus is that oxidative stress may represent the principal mediator of hyperglycemia-induced diabetic complications .
Dr Beckie Port Research Manager At Parkinson’s Uk Said:
“This study is the most robust evidence to date that there is a link between Parkinson’s and type 2 diabetes.
“The vast majority of people with diabetes will not go on to develop Parkinson’s. Studies that demonstrate type 2 diabetes is linked to an increased risk of Parkinson’s suggest, however, that the two conditions may affect cells in similar ways.
“Understanding the link between these two conditions could be key to developing treatments that slow the course of Parkinson’s, something that no current treatments can do.
“Currently, there is much interest in the potential for anti-diabetic drugs, such as exenatide, to slow the progression of Parkinson’s. The ability to repurpose these drugs, which are already used to treat people with diabetes, could speed up the delivery of new and better treatments to people with Parkinson’s who urgently need them.”
Is There A Link Between Diabetes And Parkinsons Disease
The American Academy of Neurology is the world’s largest association of neurologists and neuroscience professionals, with 36,000 members. The AAN is dedicated to promoting the highest quality patient-centered neurologic care. A neurologist is a doctor with specialized training in diagnosing, treating and managing disorders of the brain and nervous system such as Alzheimer’s disease, stroke, migraine, multiple sclerosis, concussion, Parkinson’s disease and epilepsy.
T2dm Is Associated With An Increase In The Risk Of Pd
An association between T2DM and PD was first reported by Sandyk in 1993, where it was noted that PD patients with co-existent T2DM had worse motor symptoms and reduced response to treatment . In the same study, a high prevalence of impaired glucose tolerance tests was reported among PD patients , however a more recent estimate suggests that overtly impaired glucose metabolism occurs in only around 20% .
In subsequent years, a large number of studies have explored the association between T2DM and the risk of PD. These include several prospective cohort studies which generally indicate that T2DM is associated with an increased risk of PD . For example, a large prospective study in Finland found that patients with T2DM had an 85% increased risk of developing PD and another prospective study in the US showed that patients with T2DM were 40% more likely to develop PD . A meta-analysis published in 2016 combined the effect estimates from seven population-based cohort studies and concluded that patients with T2DM had an average 28% higher risk of developing PD .
A possible explanation for the divergence observed between case-control and cohort studies is survival bias, which can be a problematic in studies with a retrospective case-control design. Higher mid-life mortality among diabetic patients could contribute towards the inverse relationship seen between T2DM and PD in these settings, and thus far has been given little consideration .
Fig. 1
Insulin Resistance Impairs Synaptic Plasticity In Pd
Dopamine depletion in PD causes changes in synaptic plasticity that result in an upregulation of factors that suppress movement and contribute to a downregulation of factors that initiate movement . The two main components of synaptic plasticity, long-term depression and long-term potentiation , are also involved in memory formation and storage through synaptic restructuring . The combined activation of mTORC1 and mTORC2 is required for dendritic regrowth, neuronal shape restructuring and synaptic plasticity via actin aggregation, for consolidating long-term memory .
Insulin promotes NMDA-mediated neurotransmission by directly activating glutamate NMDA receptors and increasing the extra-synaptic transport of GluA1 AMPA receptors in neurons involved in increasing synaptic strength and regulating LTP . A study on streptozotocin-induced diabetic rats found that NMDA and AMPA receptor expression was reduced , impairing synaptic transmission.
The common pathophysiological processes linking T2DM and PD offer new avenues for research into the use of T2DM therapeutic approaches repurposed for use in PD.
Type 2 Diabetes And The Risk Of Parkinson’s Disease
https://doi.org/10.2337/dc06-2011
The Five Environmental Causes Of Parkinsons Disease
Parkinson’s disease is a brain disorder that develops and becomes worse over time. The typical symptoms of the disease include tremor, stiffness, slowness of movement, and balance problems. These symptoms develop when the brain lost its ability to produce a sufficient amount of dopamine, a neurotransmitter responsible for controlled body movement.
Researchers have identified a variety of environmental factors that are linked to Parkinson’s disease. Some of these factors may directly cause the disease symptoms, others may increase the risk of developing it.
Here are the 5 main environmental factors that are linked to Parkinson’s disease development.
Common Diabetes Drugs May Help Prevent Parkinsons
27 October 2020
Elevated risk of Parkinson’s disease among people with type 2 diabetes appears to be reduced by some medications used to treat their diabetes, finds a new study led by UCL researchers.
The researchers are testing one of the drugs, called exenatide, as a potential Parkinson’s treatment in an upcoming clinical trial, and the new findings, published in Brain, lend support to repurposing diabetes medications for people with Parkinson’s.
The research team, funded by The Cure Parkinson’s Trust, examined patient records from 100,288 people with type 2 diabetes, from The Health Improvement Network database.
The findings confirmed that people with type 2 diabetes face an elevated risk of Parkinson’s, when compared to another cohort of people without diabetes, but commonly prescribed drugs, GLP-1 agonists and DPP4 inhibitors, appeared to reverse that relationship.
The researchers found that people who were taking two particular classes of diabetes treatments – GLP-1 agonists and DPP4 inhibitors – were less likely to be diagnosed with Parkinson’s disease a few years later , compared to people who were taking other diabetes medications. Those taking GLP-1 agonists were 60% less likely to develop Parkinson’s compared to people taking other diabetes drugs.
Overview Of Insulin Cellular Signalling Pathways
Insulin may play a key role in neuroprotection via its receptor , which activates insulin receptor substrates 1 and 2. IRS1 is particularly expressed in skeletal muscle, adipose tissue and cerebral cortex, whereas IRS2 has a particular role in the liver and the hypothalamus . Insulin binds to IR/IRS to stimulate various downstream pathways . These in turn activate downstream secondary messengers via three main pathways:
Fig. 2
Literature Search And Study Inclusion Criteria
A literature search for all English language manuscripts published up to 10 May 2011 and focused on the relationship between diabetes and PD was performed independently by E.Ce. and C.P. Queried databases were as follows: PubMed , Scopus , and Embase .
The search strategy included terms for diabetes , and PD. We also searched the references of included articles for potential additional reports. Searching queries are listed in the Supplementary Data.
Both prospective and case-control original studies investigating the role of diabetes as a risk factor for PD were potentially eligible for inclusion. Manuscripts were initially selected on the basis of the title and abstract. To be included in the quantitative analyses , articles had to report at least a risk and a precision estimate relating pre-existing diabetes to subsequent incident PD or enough data to calculate them.
Thereafter, selected studies were assessed for quality according to the following items: 1) for cohort studies, the systematic lack of case identification or bias in case ascertainment and 2) for case-control studies, the exclusion of secondary causes of parkinsonism and/or adequate comparability between PD cases and control subjects.
Common Therapeutic Approaches To T2dm And Pd
The current focus of pharmacological management of PD is to relieve symptoms by increasing circulating levodopa, inhibiting levodopa breakdown or stimulating dopamine receptors . Non-oral approaches to management are medical , surgical , or therapies-led , and they play an increasing role as the disease progresses. The current weight of evidence for therapeutic options for PD supports an effect on symptoms only and no modification of disease progression. However, recent studies on the use of T2DM drugs to treat PD have shown promising results.
