What Makes A Good Disease Model
In humans, a disease may take years to progress. Model organisms that generate the disease quickly allow scientists to study it and test potential treatments in shorter time periods. The ideal model would be an organism that is easy to monitor and manipulate in the lab, has low cost and can display the pathophysiology of the disease.
Increased Expression Of Vmat2
3-Methyl-1-phenyl-2-pyrazolin-5-one , a free radical scavenger, has demonstrated its neuroprotective effect in a chronic rotenone rat model by inhibiting ROS and apoptotic promoter Bax expression, and by enhancing the expression of VMAT2 . It obliterates rotenone-induced catalepsy, mitochondrial damage, and DA neuron degeneration in rotenone-treated rats .
Neuronal Dysfunction In Brain Regions Outside The Nigrostriatal Tract
In addition to SNpc degeneration, there is further widespread pathology in PD, reflected in cell dysfunction or loss in other subcortical brain regions such as the olfactory bulb, dorsal motor nucleus of the vagus , nucleus basalis of Meynert, the raphe nuclei, locus coeruleus and hypothalamus . These in turn encompass disruption of additional non-dopaminergic neurotransmitter systems: cholinergic, serotonergic, noradrenergic, glutamatergic, and GABAergic. Characterisation of extra-nigral degeneration remains patchy for animal models of PD so what is included below is undoubtedly incomplete. However, given the relevance of these systems for those exploring the prodromal stage or NMS of PD, these changes may become more fully characterised in the future.
LPS also appears to cause widespread dysfunction that varies depending on the route of administration. When administered intranasally, LPS induces changes in the olfactory bulb including TH+ cell reduction whereas, when administered directly in the brain, it both increases TH levels and decreases choline acetyl transferase levels in the DMV . LPS administered systemically leads to gradual degeneration of the noradrenergic neurons in the locus coeruleus of mice and neuronal loss in the hippocampus .
Recommended Reading: What Causes Dizziness In Parkinson’s
What Benefit Has Animal Research Provided To Human Health
Millions of people are alive today because of the benefits of medical research with animals, and millions more are living longer, healthier lives. Many of the treatments we take for granted today could not have been possible with animals during the research and development phase. For example, the polio vaccine, developed in part after testing on animals, has saved millions of lives after scientists developed it in the 1950s.
Autosomal Recessive Mutations: Prkn Pink1 And Dj
Autosomal recessive mutations in PRKN , PINK1, and DJ-1 have been linked to familial PD. There are over 100 known mutations of PRKN and it is the most commonly mutated gene in early onset PD . PINK1 is the second most commonly mutated gene in early onset PD, present in 17% of the cases, while DJ-1 mutations are uncommon . Rare individuals who have two mutations in one of these three genes appear to have complete penetrance .
Mutations in all three genes give loss-of-function, and therefore knockout models have been generated. However, none of the knockout models display dopaminergic cell loss or motor deficits . Some DJ-1 and PRKN models display changes in dopamine neurotransmission and mitochondrial dysfunction . Interestingly, overexpression of mutated PRKN-Q311X leads to age-dependent dopaminergic neurodegeneration, -synuclein aggregation, and some motor deficits, suggesting a gain of pathological function . Backcrossing of DJ-1 nullizygous mice onto a C57Bl/6J background generates a new PD phenotype . These mice display strong early unilateral dopaminergic neurodegeneration that progresses into bilateral pathology in 15-month-old mice, demonstrating the age- and strain-dependent nature of some PD models.
The overexpression of autosomal recessive genes, where loss-of-function is linked to PD, could become sources of treatments. For example, overexpression of PRKN via rAAV vectors had protective effects in the striatum of rAAV–synuclein-treated NHP .
You May Like: What Is The Newest Treatment For Parkinson’s Disease
Prodromal Symptoms Of Pd Patients
Prodromal symptoms are important as clinical biomarkers to identify patients in a premotor stage, especially when the fluid and imaging biomarkers are lacking. They include hyposmia, sleep abnormalities, autonomic dysfunction, and psychiatric problems, and some of the prodromal symptoms such as anxiety/depression, cognitive decline, and somnolence are much more frequent and become problematic in an advanced stage. These non-motor symptoms should be weighted as prodromal biomarkers in terms of the prevalence in PD patients, diagnostic strength to predict future development of PD, lead time to the diagnosis of PD, and feasibility of assessment and screening . The highest predictive value for the future development of PD is observed in polysomnography-confirmed RBD with a positive likelihood of 130, but their prevalence in the general population is very low and is not suitable for screening. In contrast, the predictive value of constipation is moderate with a positive likelihood of 2.5, but the prevalence in the general population is very high . Hyposmia has moderately high predictive value with a positive likelihood of 6.4, and its prevalence in the general population is moderate .
In this section we will focus on the hyposmia, constipation, and RBD, considering their importance as prodromal symptoms of PD from the viewpoints of the prevalence in PD patients, diagnostic strength to predict future development of PD, and feasibility of assessment in animals.
Emerging Genetic Models In Multicellular Model Organisms
The disappointing outcomes obtained so far by modelling the genetics of PD in mice have stimulated interest in developing alternative genetic models in multicellular model organisms. The fruitfly Drosophila melanogaster, the nematode Caenorhabditis elegans and the zebrafish Danio rerio offer some clear advantages over rodents in terms of the relative ease with which the genome can be manipulated to model the gene mutations associated with PD and of the much reduced costs involved in the development of genetic models of PD, but of course, their face validity is limited by the nature of the symptoms these species present with. Given that these models are in much earlier stages of development, they have yet to play a role in drug discovery for PD however, they may prove invaluable in the future development of disease-modifying strategies that have so far yielded little success in clinical efforts. The review would not be complete therefore without a brief mention of the promise these models hold.
Drosophila model
C. elegans model
Zebrafish model
Read Also: When A Person Is Suffering With Parkinson’s Disease
Tests Of Motor Symptoms
Animal models have been used to test novel modalities designed to primarily improve the motor dysfunction in PD. In rodents, motor function is usually assessed by measuring the following.
General Activity
1) Open field test for locomotion and 2) swim test in a water basin to observe the locomotive activity in the water .
Co-ordination
1) Rotarod test to observe motor co-ordination, in which a rat or mouse is placed on a rotating rod, and the time for which the animal can hold before falling down is measured . 2) Cylinder test to analyze the loss of voluntary forelimb movement. This is especially useful in evaluating unilateral 6-OHDA-injected rats or mice, and also in some genetic models. It is also used to rate the LIDs in DA-depleted mice . 3) Pole test to observe the time taken by the rodent models placed on the top of a vertical pole to reach the floor is used to assess the motor activity . 4) Challenging beam traversal test to detect subtle deficits in motor skills, motor coordination, and balance .
Gait Performance
1) Forepaw stride length test is used to analyze the gait abnormalities in rodents as they walk in a straight line on a white paper with their feet covered with black ink . 2) A grid test is used to assess the coordination in rodents by measuring the number of footslips of a given limb during free exploration of the grid .
Webinar: The Search For Clinically Relevant Motor Behaviors In Animal Models Of Parkinsons Disease
Dr. Robert Hodgson from Charles River Laboratories talks in a webinar about the evolution of equipment for the exploration of the motor deficits in animal models with Parkinsons Disease and the methods available for the study of kinematic gait analysis, such as the MotoRater.
What is the Motorater?
MotoRater is a system for kinematic gait analysis for mice and rats with high sensitivity. It evaluates different motion modalities: overground walking, skilled ladder walking, wading in water and swimming. Additionally, the MotoRater allows testing in water, which helps evaluate impaired rodent models.
What disease models can be researched with the MotoRater?
The following disease models can be analyzed: stroke, Huntigtons Disease, Parkinsons Disease, multiple sclerosis, amyotrophic lateral sclerosis, Batten disease, Duchenne muscular dystrophy, and other rate diseases.
Check the MotoRaterpage for further information about the system, scientific publications, and brochure.
You May Like: Does Gabapentin Help Parkinson’s
Models Of Autosomal Recessive Pd
Mutations in thePRKN gene account for more than 50 % of early-onset familial PD cases and at least 20 % of early onset sporadic PD, also referred to as PARK2 PD . Mutations in DJ-1, PINK1, FBX07, ATP13A2, and PLA2G6 are less common forms of autosomal recessive PD . So far, only PRKN, DJ-1, and PINK1 genetic mice models are available for the study of PD.
PRKN Gene Model
DJ-1 Gene Model
PINK1 Gene Model
Mutations in PINK1 cause another autosomal recessive form of PD . PINK1 knockout mice do not exhibit major abnormalities in the DA neurons or striatal DA levels only mild mitochondrial and nigrostriatal neurotransmission deficits may be present, associated with increased susceptibility to oxidative stress and ROS production . PINK1G309D transgenic mice have an age-dependent moderate reduction of DA levels accompanied by low locomotor activity .
Experimental Diabetes Drug Shows Promise In Parkinsons Disease
The drug, MSDC-0160, offers a new approach to treating Parkinsons disease, targeting the underlying disease rather than alleviating symptoms.
Drug discovery and development
Shutterstock
An experimental drug originally developed for type 2 diabetes has shown early promise as a treatment for Parkinsons disease, say researchers who are now planning a clinical trial in humans.
Known as MSDC-0160, the drug differs from current treatments for Parkinsons disease in that rather than simply alleviating symptoms, it is designed to target the underlying disease.
Study leader Patrik Brundin, director of the Van Andel Research Institutes Center for Neurodegenerative Science, Michigan, hopes the study will be a watershed moment for Parkinsons disease research.
All of our research in Parkinsons models suggests this drug could potentially slow the diseases progression in people as well, he says.
He adds that, if successful in human trials, MSDC-0160 would be the first therapy in Parkinsons disease to treat the underlying disease and potentially slow its progression.
Several studies have shown potential similarities in metabolic changes at the molecular level in diabetes and Parkinsons disease. This has led to an increased interest in the effects of thiazolidinediones a group of insulin sensitising drugs in patients with Parkinsons disease, the US researchers report in Science Translational Medicine .
Dont Miss: Scan To Diagnose Parkinsons
Also Check: What Disease Has The Same Symptoms As Parkinson’s Disease
Therapeutic Strategies For Lids In Animal Models Of Pd
Chronic administration of levodopa is often compromised by numerous side effects, such as LIDs in PD patients . Modulation of DA, glutamate,adenosine, and other systems has been explored in various animal models of LIDs .
DA Receptor Antagonists
To address the question whether inhibition of various DA receptors can be used to treat LIDs, selective D1, D2, and D3 DA receptor antagonists have been tested in 6-OHDA-lesioned rat model with LIDs and found that these antagonists are able to inhibit the LIDs without disrupting the therapeutic potential of levodopa . This result suggests that the anti-parkinsonian effects of levodopa and its dyskinetic side effects are mediated through different DA pathways.
N-Methyl-D-Aspartate Receptor Antagonists
Mixed N-methyl-D-aspartate receptor antagonist and serotonin agonist amantadine and dextromethorphan have proven to be effective in attenuating LIDs in PD patients and LIDs in 6-OHDA rat model . However, pure NMDA agonists like dizocilpine show no effect on suppressing LIDs in low doses when used in a parkinsonian rat model . But when used in high doses, they reduce the LIDs and also suppress levodopa-induced contralateral rotations . In a clinical trial conducted on PD patients, the use of NMDA receptor 2B selective NMDA glutamate antagonist CP-101,606 improves LIDs, but left patients with side effects, such as dose-dependent dissociation and amnesia .
AMPA Receptor Antagonists
Metabotropic Glutamate Receptor Modulators
Study To Identify Clinical Imaging And Biologic Markers Of Parkinson Disease Progression
Sorry, in progress, not accepting new patients
This is a observational, multi-center study to assess progression of clinical features, imaging and biologic biomarkers in Parkinson disease patients compared to healthy controls and in PD patient subtypes. The primary objective of this study is to identify clinical, imaging and biologic markers of PD progression for use in clinical trials of disease-modifying therapies.
La Jolla, California and other locations
The study, funded by Parkinsons UK, suggests that the drug, tasquinimod, which is not yet on the market, works by controlling genes that may cause Parkinsons. This happens when the drug interacts with a protein inside brain cells.
The team at the Oxford Parkinsons Disease Centre used cutting-edge stem cell techniques to grow brain cells from skin cell samples donated by people with a rare genetic form of Parkinsons, and from healthy people without the condition.
The team followed the progression of the condition in brain cells made from the patients stem cells and saw that a number of important genes became inactive when problems first started to occur inside the Parkinsons-inflicted cells.
The switching off of these genes early in the process brought the condition on later.
Don’t Miss: Do You Have Hallucinations With Parkinson’s
What Sort Of Non
Animals are used in research only when no other adequate methods are available. Researchers at The Neuro use many techniques to study the brain that do not involve animals. Induced pluripotent stem cells obtained from individual patients are a powerful source of neural cells for personalized medicine. Non-invasive cognitive testing on humans, for example, helps us understand basic brain functions that can inform future studies into neurological problems. Other scientists use artificial intelligence and big datasets to find patterns in disease pathology. This can help us understand how diseases such as Alzheimers and Parkinsons progress, and how we can best stop them. Genetic testing using human samples also tells us about the role of genes in neurological disease and how we can target specific gene pathways to treat or prevent diseases.
Recent Daergic Drug Studies On Animal Models Of Pd
Intranasal Levodopa
Certain motor tests like turning behavior in an open field, foot slips on a horizontal grid, and postural motor asymmetry in a cylinder may be assessed after intranasal levodopa administration with and without benserazide in a unilateral 6-OHDA-lesioned rat model . Intranasal levodopa treatment reduces ipsilateral turning, and contralateral forelimb slips on the grid . These results suggest that intranasal levodopa can bypass the BBB and attenuate the motor impairments in the parkinsonian 6-OHDA-lesioned rat model.
Sustained-Release Formulation of Levodopa Methyl Ester/Benserazide
When administered in the form of microspheres into 6-OHDA-lesioned rat model, levodopa methyl ester/benserazide achieves sustained release and markedly attenuates apomorphine-induced rotations and rectifies the imbalance steps of the parkinsonian rats .
Transdermal Rotigotine Patch
Non-ergot DA agonist rotigotine can be used as a single drug or in combination with levodopa . Continuous transderma delivery of rotigotine has been shown to provide true constant DAergic stimulation in MPTP animal model of PD . Rotigotine has been found to be effective in early, as well as advanced PD, to treat early morning and nocturnal motor symptoms, and also in non-motor symptoms such as sleep disturbances, depression, and pain .
Continuous Subcutaneous Infusion of Ropinirole via Osmotic Minipumps
Don’t Miss: Voice Amplification Devices For Parkinson’s
Animal Models Of Pd: A Brief Overview
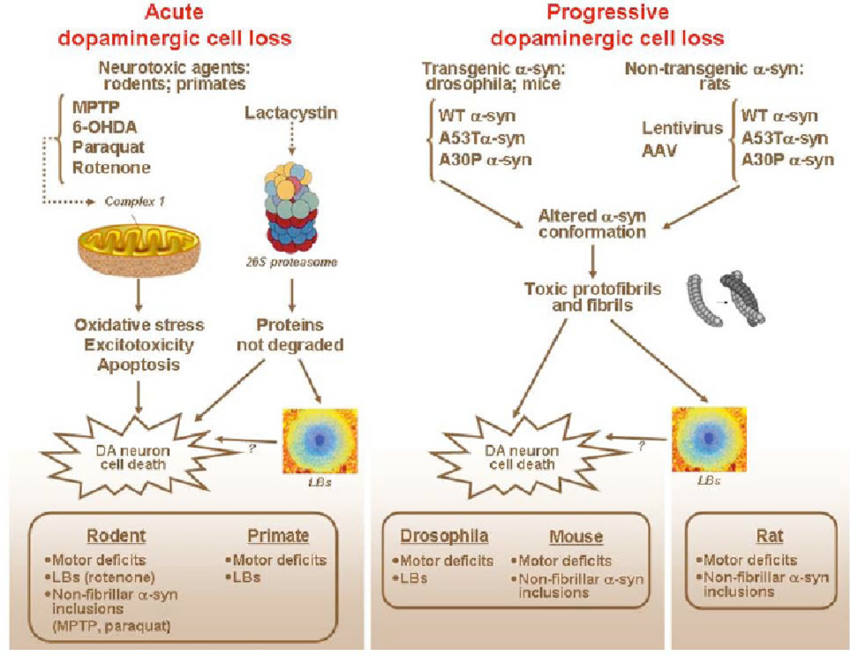
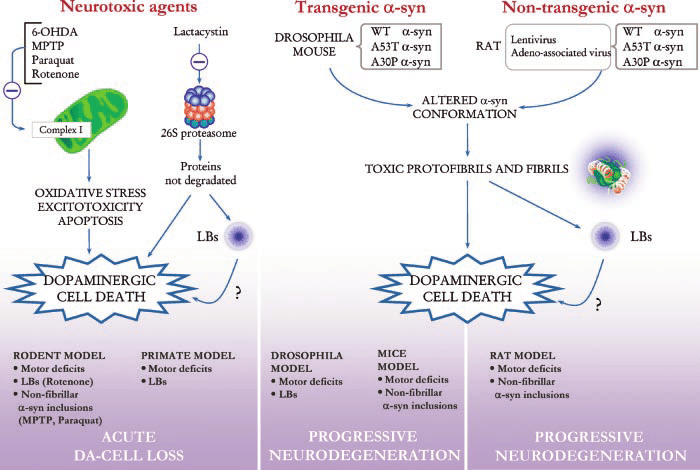
In contrast with the situation for many other neurodegenerative diseases, PD benefits from of a wide range of available animal models, the different classes of which are briefly summarised below. We have focused here on the mammalian models readers interested in the various non-mammalian models such as those in Drosophila melanogaster or Caenorhabditis elegans are directed towards existing reviews .
For more permanent effects, toxin models have been developed. These toxins can be broadly subcategorised into neurotoxins , pesticides and endotoxins . The mechanism for how each of these toxins cause their degenerative effects is reviewed in detail elsewhere . In brief, when administered intracranially into the nigrostriatal tract or systemically , they mostly cause disruption of mitochondrial complexes involved in oxidative phosphorylation, alongside an increase in reactive oxidative species and ultimately nigral cell death. LPS, on the other hand, is thought to induce a PD-like phenotype through enhancing microgliosis and local iron and ferritin levels at the site of injection . Typically, these toxin models induce a rapid loss of dopaminergic cells that gives rise to motor dysfunction and further behavioural deficits.
Modifications Of The Classical Neurotoxin
Up-to-date, development of the next generation drugs for PD that aim to stop or slow down the disease progression is unlikely due to the lack of PD models that truly reflect the widespread and progressive pathology of the illness and its complexity. Similarly, little progress has been made in moving into other pharmacological areas for the treatment of PD. There is no doubt that the availability of experimental animal models of PD has hugely altered dopaminergic drug treatment of the motor signs of the PD as well as improved the prevention and reversal of drug related side effects that emerge during the disease progression .
An extensive pharmacokinetic, pharmacodynamic and pathophysiological data is required to establish an entirely new animal model of a disease. Thus, it is much easier to modify existing experimental models that are known to form the hallmarks of the PD process, and indeed, such attempts have been undertaken. The most promising attempts may be classified into the following categories: 1) neurotoxin-based models with improved protocols, mostly in terms of prolonged delivery or additional routes of administration 2) administration of well-studied neurotoxins to genetically modified animals 3) models of dopamine and L-DOPA-related neurotoxicity.
You May Like: What Causes Parkinson’s Tremors
