Etiology Of Parkinson’s Disease
Thomas Gasser, … Mahlon R. DeLong, in, 2015
Etiology of Parkinson Disease: Clues from Epidemiology and Genetics285
-
Epidemiology and Environmental Risk Factors285
-
Twin Studies285
-
Genetic Causation of Parkinson Disease286
-
Autosomal Dominant Forms of Parkinson Disease286
-
Autosomal Recessive Forms of Parkinson Disease287
-
Genetic Risk Factors for Sporadic Parkinson Disease288
-
Genetic Models for Parkinson Disease288
-
Selective Neuronal Degeneration in Parkinson Disease289
-
Pathology and Pathogenesis289
-
Relationship between Parkinson Disease and Multiple Systems Atrophy290
-
Pathophysiology291
-
Pattern of Dopamine Loss in the Striatum293
-
Discharge Rate Changes in the Basal Ganglia293
-
Changes in Activity Patterns of Basal Ganglia Networks294
-
Relationship of Rate and Pattern Changes to Parkinsonism294
-
Role of Non-Motor Basal Ganglia Circuits in Parkinson Disease295
Roger C. Duvoisin, in, 1981
Genetics: Insights Into Etiology
Improvement in genetic analysis techniques in the 1990s led to the discovery of the first genetic cause of PD: mutations in the SNCA gene encoding -synuclein . At around the same time, -synuclein was found to be the major constituent of LB, the pathological hallmark of PD . Subsequently, multiplications of the SNCA gene have been found to cause PD with penetrance increasing with gene dosage . These discoveries brought -synuclein to center stage in the study of the pathogenesis of PD and led to the hypothesis that during different stages of the disease, -synuclein spreads in a stereotypical way within the nervous system in a prion-like fashion .
Table 2 Examples of genes associated with PD risk
Pathogenesis Of Parkinsons Disease
A number of mechanisms have been implicated in PD pathogenesis, with -synuclein aggregation central to the development of the disease. Multiple other processes are thought to be involved, with several studies suggesting that abnormal protein clearance, mitochondrial dysfunction, and neuroinflammation play a role in the onset and progression of PD. However, the relationship between these pathways remains unclear.
Don’t Miss: Can Parkinson’s Disease Be Fatal
The Role Of Neuroinflammation On The Pathogenesis Of Parkinson’s Disease
- Chung, Young-Cheul
- Ko, Hyuk-Wan
- Bok, Eu-Gene
- Park, Eun-Soo
- Huh, Sue-Hee
- Nam, Jin-Han
- Jin, Byung-Kwan
Abstract
Parkinson’s Disease is a common neurodegenerative disease characterized by the progressive degeneration of nigrostriatal dopaminergic neurons. Although the causative factors of PD remain elusive, many studies on PD animal models or humans suggest that glial activation along with neuroinflammatory processes contribute to the initiation or progression of PD. Additionally, several groups have proposed that dysfunction of the blood-brain barrier combined with infiltration of peripheral immune cells play important roles in the degeneration of DA neurons. However, these neuroinflammatory events have only been investigated separately, and the issue of whether these phenomena are neuroprotective or neurotoxic remains controversial. We here review the current knowledge regarding the functions of these neuroinflammatory processes in the brain. Finally, we describe therapeutic strategies for the regulation of neuroinflammation with the goal of improving the symptoms of PD.
Keywords
References
Cited by
Molecular Convergence And Divergence
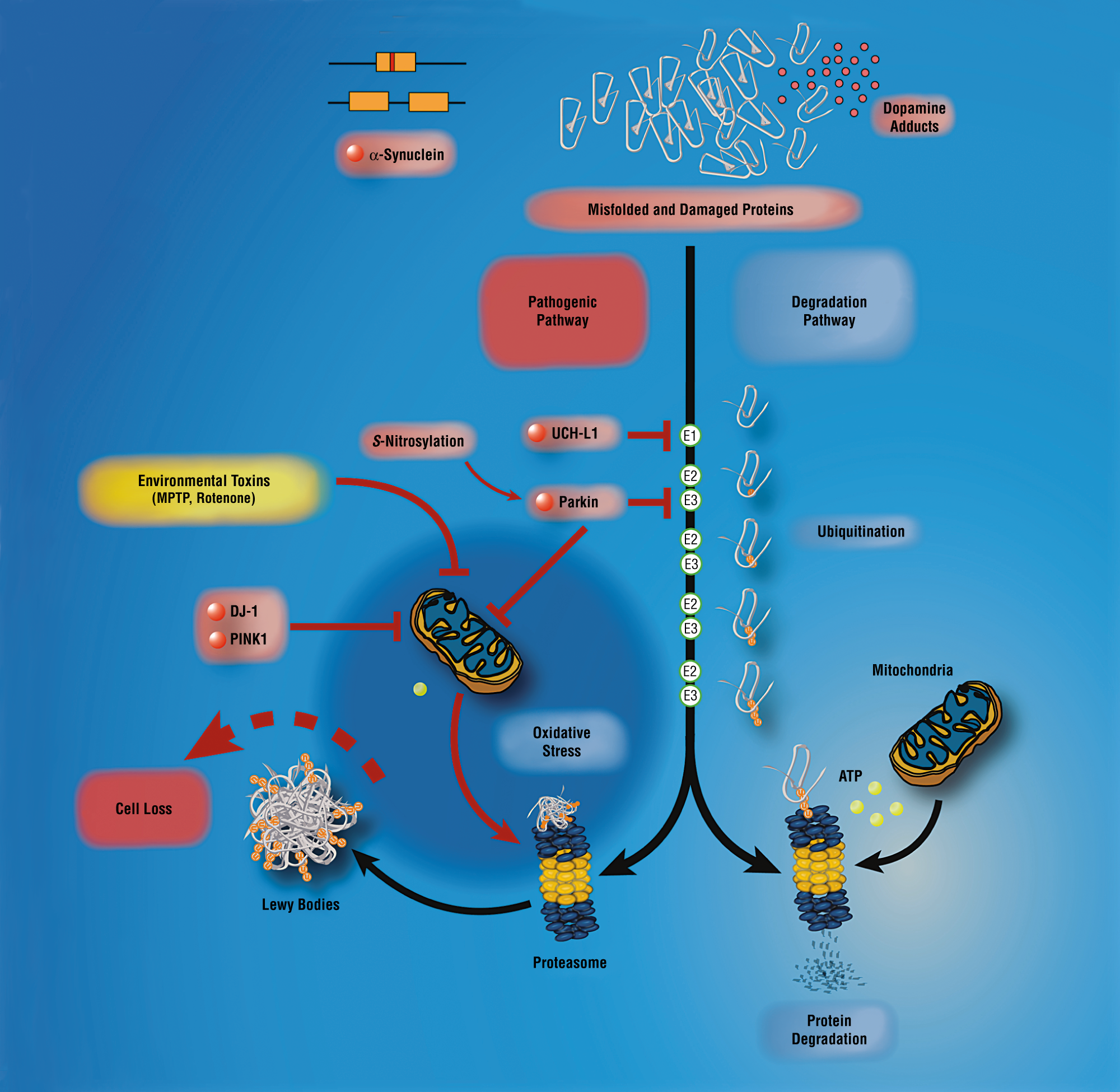
The inherited forms of parkinsonism demonstrate how a single molecular aberration is sufficient to independently reproduce the clinical and pathological features of PD. Moreover, the mutation is present in all cells of the body, and yet, the cell loss is restricted to the substantia nigra and associated structures, informing that these molecular events must be cell specific to precipitating PD-type neurodegeneration. However, with the growing wealth of genetic clues, comes increasingly complex puzzles: how can one reconcile the fact that the PD phenotype can occur in the absence of Lewy bodies and yet a biochemical excess of normal alpha-synuclein as a result of alpha-synuclein gene triplication is also sufficient to cause PD? Is it possible to place the UPS, mitochondrial function, oxidative stress, protein aggregation and kinase signalling in one unified pathway that leads to nigral cell death? Furthermore, if there are instead several distinct pathways that are separately able to precipitate nigral cell death, which of these is the most significant in sporadic PD?
You May Like: Does Parkinson’s Disease Hurt
Current Trends In Pd: Less Alpha
The notions that prion-like spreading of misfolded alpha-synuclein causes PD and that the gut is a site of origin of the disease have both received a great deal of attention. Although tempting and attractive in their simplicity, these hypotheses are nevertheless contentious and still widely debated. Based on existing findings obtained in human tissues and in animal models, we have recently reviewed the arguments for and against the gut as a potential starting point for the disease and we
Pd In The Nineties And Noughties: Alpha
Alpha-synuclein, a 143 amino acids protein was first isolated and sequenced in 1988 from the electric organ of the Pacific electric ray Torpedo californica. In addition to its strong presynaptic localization, Maroteaux et al., also identified alpha-synuclein in the nucleus, thus accounting for the name synuclein . Its 140 amino acids human and rat homologues were subsequently sequenced , . These first observations on alpha-synuclein found
Recommended Reading: Is Leg Pain A Symptom Of Parkinson’s Disease
The Interplay Between Glia And Peripheral Immune Cells In Parkinson’s Disease
Glial cells are crucial to the maintenance of homeostasis within the CNS and the disruption of this is linked to numerous CNS diseases. There appears to be little evidence available outlining potential interactions between peripheral immune cells and glia in PD, however the research into this area is increasing and is summarised in Figure 1. A recent study demonstrated antigen presenting capabilities in astrocytes. MHCII expressing astrocytes were identified in close proximity to CD4+ T cells in the post-mortem brain tissue of PD patients and cultured human astrocytes exposed to pre-formed fibrils of -syn expressed the T cell co-stimulatory structures, CD80, CD86, and CD40 , suggesting the capacity to activate CD4+ T cells. Although Rostami et al., determined that cultured human microglia demonstrated poor antigen presenting capabilities, Harms et al., determined in mice that microglia exposed to -syn increased their expression of MHCII, became activated and induced proliferation of CD4+ T cells. Furthermore, the knockout of MHCII prevented microglial activation and dopaminergic cell loss in these mice .
Inflammation In The Pathogenesis Of Parkinsons Disease
ABSTRACT: The immunohistochemical demonstration of reactive microglia and activated complement components suggests that chronic inflammation occurs in affected brain regions in both Parkinsons disease and Alzheimers disease. Chronic inflammation can damage host cells, and there is epidemiological evidence that it contributes to the progressive neuronal loss in Alzheimers disease. Reports in the literature indicate that anti-inflammatory agents inhibit dopaminergic cell death in animal models of Parkinsons disease. There is a marked elevation in the levels of the messenger ribonucleic acids for complement proteins and markers of activated microglia in affected regions in both Parkinsons disease and Alzheimers disease. The upregulation appears greater than that found in inflamed arthritic joints. These data support the hypothesis that chronic inflammation may play an important, if secondary, role in the pathogenesis of Parkinsons disease.
The authors present data to support the hypothesis that inflammation may play an important role in the pathogenesis of Parkinsons disease.
Reactive microglia are also seen in the basal ganglia in 6-hydroxydopamine and MPTP animal models of PD, and there are several reports that anti-inflammatories inhibit dopaminergic neurotoxicity in such animal models.
Don’t Miss: What Area Of The Brain Does Parkinson’s Disease Affect
Impact Of Parkinson’s Disease Related Genetics On Astrocyte Function
There are monogenic mutations identified in 20 genes that have been implicated in the pathogenesis of PD . Interestingly, a study by Zhang et al. compared the transcriptome of human astrocytes to neurons, and found upregulation of some of these monogenic mutations in astrocytes was to a similar level and sometimes higher than that of neurons . This would strongly support the potential contribution of astrocytes to the pathogenesis of these familial forms of PD. Altered levels of these genes lead to many changes in astrocyte function including impaired glutamate uptake, liposomal homeostasis, lysosomal, and mitochondrial dysfunction and inflammatory response .
Table 1. The role of genes that are causative in Parkinson’s disease pathogenesis and their implications in astrocytes.
DJ-1/PARK7
PARK2 and PINK1
SNCA
LRRK2 and GBA
Other Parkinson’s Disease Risk Genes
The Sources Of Reactive Oxygen Species And Its Possible Role In The Pathogenesis Of Parkinsons Disease
Cheng-wu ZhangLin Li
1Key Laboratory of Flexible Electronics & Institute of Advanced Materials , Jiangsu National Synergetic Innovation Center for Advanced Materials , Nanjing Tech University , 30 South Puzhu Road, Nanjing 211816, China
2Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore
Abstract
Parkinsons disease is the second most common neurodegenerative disorder characterized by progressive loss of dopaminergic neurons in the substantia nigra. The precise mechanism underlying pathogenesis of PD is not fully understood, but it has been widely accepted that excessive reactive oxygen species are the key mediator of PD pathogenesis. The causative factors of PD such as gene mutation, neuroinflammation, and iron accumulation all could induce ROS generation, and the later would mediate the dopaminergic neuron death by causing oxidation protein, lipids, and other macromolecules in the cells. Obviously, it is of mechanistic and therapeutic significance to understand where ROS are derived and how ROS induce dopaminergic neuron damage. In the present review, we try to summarize and discuss the main source of ROS in PD and the key pathways through which ROS mediate DA neuron death.
1. Introduction
In this review, we will focus on discussing how the PD-associated factors induce ROS generation and how ROS lead to dopaminergic neuron death in PD .
2. ROS and PD-Associated Factors
2.1. PD-Related Genetic Mutations and ROS
Read Also: What Is The Main Cause Of Parkinson Disease
An Emerging Role For The Peripheral Immune System In Parkinson’s Disease
Evidence of an important role for inflammation in the pathogenesis of PD is emerging and the data suggests that peripheral immune cells may contribute to this inflammation. In this section of the review we cover the latest studies demonstrating a role for the peripheral immune system and in particular T cells in PD. By way of introduction however a brief explanation of innate and adaptive immunity is outlined below.
Environmental Factors: Etiological And Disease
From 1817 when Dr. James Parkinson first described 6 patients with the condition that would later bear his name , much progress was made in the following 150years. It became known that the SN was the site affected by PD pathology together with the presence of cytoplasmic inclusions, and levodopa became available as the first effective symptomatic drug treatment of PD in 1960s . However, the etiology of PD remained elusive.
Table 1 Examples of environmental factors and their biologic correlates
The most robust beneficial environmental factor associated with PD is cigarette smoking which has been seen in early case-control studies and then confirmed in more recent large prospective cohorts. Active smokers have 50% lower risk of PD compared to never smokers . There is a strong dose-response relationship including duration, intensity, pack-years and years since last smoking: PD risk decreases with increasing duration of smoking and increases again with time since quitting . Preliminary reports also support an inverse relationship between passive smoking, smokeless tobacco use and PD .
Also Check: How To Identify Parkinson’s Disease
Pathophysiology Of Parkinsons Disease
Although we are learning more each day about the pathophysiology of Parkinsons disease, it is still considered largely idiopathic . It likely involves the interaction of host susceptibility and environmental factors. A small percentage of cases are genetically linked and genetic factors are being intensely studied.
Physiologically, the symptoms associated with Parkinsons disease are the result of the loss of a number of neurotransmitters, most notably dopamine. Symptoms worsen over time as more and more of the cells affected by the disease are lost. The course of the disease is highly variable, with some patients exhibiting very few symptoms as they age and others whose symptoms progress rapidly.
Parkinsons is increasingly seen as a complex neurodegenerative disease with a sequence of progression. There is strong evidence that it first affects the dorsal motor nucleus of the vagus nerve and the olfactory bulbs and nucleus, then the locus coeruleus, and eventually the substantia nigra. Cortical areas of the brain are affected at a later stage. Damage to these various neuronal systems account for the multi-faceted pathophysiologic changes that cause impairments not just to the motor system but also to the cognitive and neuropsychological systems .
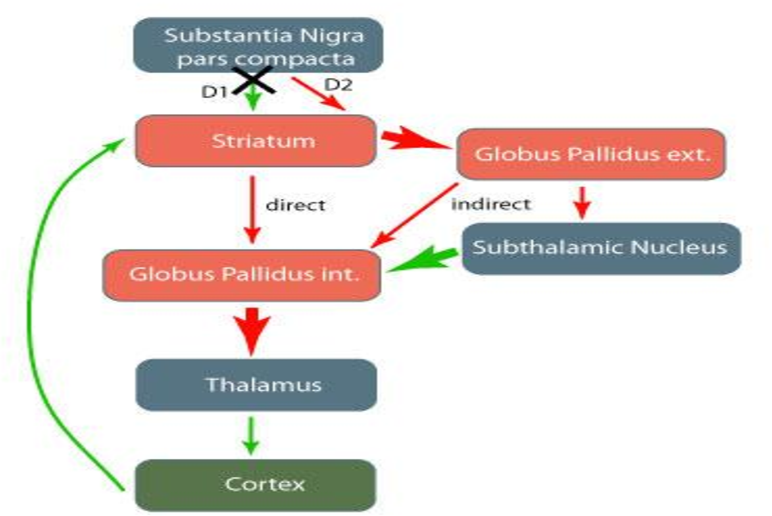
Motor Circuit In Parkinson Disease
The basal ganglia motor circuit modulates the cortical output necessary for normal movement .
Signals from the cerebral cortex are processed through the basal ganglia-thalamocortical motor circuit and return to the same area via a feedback pathway. Output from the motor circuit is directed through the internal segment of the globus pallidus and the substantia nigra pars reticulata . This inhibitory output is directed to the thalamocortical pathway and suppresses movement.
Two pathways exist within the basal ganglia circuit, the direct and indirect pathways, as follows:
-
In the direct pathway, outflow from the striatum directly inhibits the GPi and SNr striatal neurons containing D1 receptors constitute the direct pathway and project to the GPi/SNr
-
The indirect pathway contains inhibitory connections between the striatum and the external segment of the globus pallidus and between the GPe and the subthalamic nucleus striatal neurons with D2 receptors are part of the indirect pathway and project to the GPe
The STN exerts an excitatory influence on the GPi and SNr. The GPi/SNr sends inhibitory output to the ventral lateral nucleus of the thalamus. Dopamine is released from nigrostriatal neurons to activate the direct pathway and inhibit the indirect pathway. In Parkinson disease, decreased striatal dopamine causes increased inhibitory output from the GPi/SNr via both the direct and indirect pathways .
Recommended Reading: Are Headaches Associated With Parkinson’s
Selective Vulnerability Of The Nigrostriatal Dopamine Neuron
In PD, dopamine neurons within the substantia nigra are considered a selectively susceptible population of cells, whereas the adjacent dopamine neurons of the ventral tegmental area are much more resistant to degeneration. The vulnerability of the nigrostriatal neurons is due to several factors, including their unique anatomy, physiology, bioenergetic profile, and neurochemistry . First, in rat brain, the length of the axon arbor of a single nigrostriatal neuron is up to 80 cm in humans, this is estimated to be 4 m! In the rat, a single nigrostriatal neuron makes 100000240000 synapses in the striatum. In humans, it is estimated that a nigrostriatal neuron makes 10000002400000 synapses. By contrast, the VTA neuron makes about 10-fold fewer synapses and therefore has a much lower bioenergetic demand. Further compounding the bioenergetic demand of the nigrostriatal neuron is the fact that their axons are unmyelinated. Thus, propagation of each action potential and subsequent repolarization requires much more energy than if the fibers were myelinated.
Pathogenic Protein Function In Autoimmunity
As discussed above, molecular mimicry and cross immunoreactions are two of the primary mechanisms through which autoimmunity is triggered. Molecular mimicry between herpes simplex virus 1 and human -syn was detected in PD patients in 2016. HSV1 infection could enhance the development of autoimmunity because autoreactive antibodies induced by HSV1 have the same response to the human -syn homologous peptide bound to the membrane of DNs and lead to DN destruction . These results also support the assumption that -syn participates in autoimmunity involved in the pathological progression of PD.
Read Also: Does Keto Help Parkinson’s Disease
Mitochondrial Dysfunction In Pd
1.3.2.1Mitochondrial DNA Damage
The association of mutations in genes regulating mitochondrial function and PD is further supported by the regulation of mitochondrial DNA . Mitochondrial DNA encodes 37 genes, including 13 protein subunits for complex I-V of the electron transport chain . mtDNA is located in association with the inner mitochondrial membrane, neighboring where oxidative phosphorylation occurs. Therefore, it is constantly exposed to ROS.53 The lack of histones and limited repair mechanisms causes mtDNA to be especially vulnerable to damage, such as strand breaks and base modifications these in turn can lead to mutations.54 Accordingly, mutations have been found at a higher rate in dopamine neurons of the substantia nigra in PD cases than age-matched controls, suggesting a role for mtDNA mutations in the pathogenesis of PD.5558
1.3.2.2Complex I Inhibition
Vstrategies For Gene Therapy Intervention In Pd
The multi-factorial aetiology of PD means that intervention in the disease process via gene therapy is possible on many different levels, with several distinct strategies currently being investigated. First, transfer of genes involved in dopamine biosynthesis could help with the immediate motor symptoms of the disease by sustained local production of dopamine in the same way as systemic administration of oral l-dopa. Second, transfer of growth factor genes such as glial cell line-derived neurotrophic factor could restore atrophic neurons to a normal state and promote reinnervation of the damaged nigrostriatal system and prevent further dopaminergic cell death . Third, transfer of genes involved in inhibitory neurotransmission could be used to dampen the activity of brain nuclei that become overactive in PD. These three main approaches have currently reached pre-clinical primate studies or Phase I human clinical trials, based largely on demonstration of efficacy in rodent and primate toxin models of PD such as 6-OHDA and MPTP. However, the identification of genetic components in PD pathogenesis has resulted not only in development of new PD animal models, but in identification of novel targets for PD gene therapy .
C. Cebrián, … D. Sulzer, in, 2014
THOMAS N. CHASE, M. MARAL MOURADIAN, in, 1994
Also Check: How Do You Beat Parkinson’s Fatigue
