Findings On Medication Use And Motor Complications
The results showed that deep brain stimulation was effective at reducing motor complications from Parkinsons disease for more than 15 years.
The researchers also found that the therapy reduced the amount of time a person experienced dyskinesia by a whopping 75%. Dyskinesia is a side effect of a common Parkinsons medication called levodopa that results in rapid, involuntary body movements, like twisting, squirming, and head bobbing.
Whats more, participants spent about 59% less time in an off state, when medication stopped being as effective, and reduced their use of medications to manage dopamine levels by 51%.
Overall Results Show Dbs Superior To Medical Therapy
The results showed that patients who received DBS had a 26% improvement in quality of life scores compared with no improvement in the medical treatment group. There was a similar result for social functioning.
The results also showed that compared to medical therapy, DBS was significantly superior to medical treatment with respect to motor disability, activities of daily living, levodopa-induced motor complications, and time with good mobility and no dyskinesia.
Among adverse side effects, suicide or attempts at suicide were not very different in the two groups, suggesting the cause lies with the patients rather than the type of treatment, something that is important to take into account in patient counseling, note the authors.
Deuschl says in a statement:
The study showed surprisingly homogeneous results in favor of DBS compared with medical treatment.
The most important result is that quality of life of these patients and their social functioning was significantly improved. It is also meaningful that the operation has fewer side effects in this younger population than in advanced disease, he adds.
Parkinson’s Patients Saw Continued Improvement In Motor Symptoms Quality Of Life
byJudy George, Senior Staff Writer, MedPage Today June 2, 2021
Deep brain stimulation remained effective in Parkinson’s disease patients more than 15 years after the device was implanted, and patients continued to demonstrate significant improvement in motor symptoms, a retrospective study showed.
Parkinson’s patients who had bilateral subthalamic nucleus deep brain stimulation for 15 years or longer spent 75% less time with dyskinesia and 58.7% less time in the off state than pre-surgery baseline , reported Elena Moro, MD, PhD, of Grenoble Alpes University in France, and co-authors.
These patients also reduced their dopaminergic drugs by 50.6% , they wrote in Neurology. The Parkinson’s Disease Quality of Life questionnaire total score, emotional function domain score, and social function domain score improved by 13.8% , 13.6% and 29.9% , respectively.
“Deep brain stimulation benefits seem to last for several years but not enough data have been available to show that these effects are still present more than 15 years after surgery,” Moro said in a statement.
“Our study found that, despite the natural progression of Parkinson’s disease and the worsening of some symptoms that become resistant to medications over the years, participants still maintained an overall improvement in quality of life,” she added.
Disclosures
Read Also: Can Parkinson’s Cause Dementia
What Is Deep Brain Stimulation And How Does It Work
DBS is a therapy that we have for various neurological conditions, said Dr. Sheth. It’s a system that you can think of like a pacemaker. But rather than being a pacemaker for the heart, it’s for the brain.
Dr. Sheth describes the brain as having many circuits that govern everything we do, including how we move.
If the movement circuit is not working properly, we may have a movement disorder like Parkinson’s, he said. If we can identify the circuit within the brain that is not working properly, we can use this device to reset the rhythms in the brain and restore the balance so that our movements can be better controlled or without a tremor.
Candidates For Deep Brain Stimulation
The Parkinsons and Movement Disorders team meets weekly to review patient cases, including patients who may be candidates for the deep brain stimulation procedure. Patient selection is based on a thorough analysis of their medical situation and needs, as well as the best evidence available in medical literature and our extensive experience in performing DBS procedures.
Possible candidates for DBS include:
- Patients with Parkinson’s disease, essential tremor and dystonia who experience movement-related symptoms that cannot be controlled by medications.;
- Patients who have had an adequate and reasonable trial of medications
- Patients who experience intolerable side effects from medication may also be candidates.;
New uses for DBS are being investigated, including symptom control for patients with epilepsy, Tourettes syndrome, depression and chronic pain syndromes.
DBS has been successful in treating patients as young as 13 years old. In general, surgery is performed on patients under 75 years old, but this is not a firm guideline. Each patient must be assessed individually in regard to his or her stamina and overall health.
Thanks to significant innovation in DBS therapy, we offer three FDA-approved DBS devices. You and your movement disorder neurologist will thoroughly discuss;the differences between the three options;and determine which one meets your needs.
Read Also: Is Parkinson’s Disease Fatal
Why A Doctor May Choose Deep Brain Stimulation
According to the National Parkinson Foundation, the ideal Parkinsons disease candidate for DBS surgery has:
-
PD symptoms that interfere with activities of daily living.
-
Fluctuations in mobility due to PD medications with or without dyskinesia .
-
Continued good response to PD medications, even if the medication effects may wear off sooner than they have in the past.
-
A history of several different combinations of PD medications while under the supervision of a neurologist specializing in movement disorders.
These factors* may make a person a less than ideal candidate for DBS surgery:
-
Difficulty with balance, walking, or freezing as the main disabling symptom.
-
A primary symptom of speech difficulty.
-
Continuous confusion and problems with memory and thinking.
-
A psychiatric condition such as depression or anxiety that has not improved or stabilized with other treatment.
-
Another condition that increases the risk for surgery complications.
*Some of these factors may be treatable. Having one or more does not disqualify a person for future DBS surgery, but the doctor may recommend more aggressive therapy focused on these issues before surgery takes place.
What You Need To Know
- Surgeons implant one or more small wires in the brain during a surgical procedure.
- The leads receive mild electrical stimulation from a small pulse generator implanted in the chest.
- Proper patient selection, precise placement of the electrodes and adjustment of the pulse generator are essential for successful DBS surgery.
- DBS does not fully resolve the symptoms of PD or other conditions, but it can decrease a patients need for medications and improve quality of life.
Recommended Reading: Can Alcoholism Mimic Parkinson\’s
Deep Brain Stimulation Surgery And Implantation
DBS consists of two surgeries, spaced approximately three to six weeks apart to ensure the patient has adequate time to recover. Throughout your experience, you will be attended to by a top team of physicians and other medical experts including a neurosurgeon, an electrophysiologist, and an anesthesiologist.
It should be noted that DBS offers many benefits. The generator can be programmed by a neurologist, and customized to each individual patient. The procedure is also reversible. Most patients experience a significant improvement of symptoms. However, as with any brain surgery, there are risks. With DBS, the risk of stroke is 1 in 100 and infection is 1 in 50.
Today, many more patients could be helped by DBS than are currently benefiting from the procedure. Statistics show only 7 percent of Parkinsons disease and 1 percent of tremor patients in Michigan who would benefit from the procedure have undergone DBS. At U-M, we are proud to have one of the superior DBS programs in the country. We have developed a wide array of ways to improve DBS, including special imaging tools that help doctors more accurately place the electrodes, and lead intraoperative motor and speech testing that result in fewer side effects for the patient.
U-M is also home to an active research program, where our team of experts is always working on ways to make DBS faster and more accurate. We also regularly have clinical trials available for patients interested in participating.
Who Is A Candidate For Deep Brain Stimulation
DBS is more than just a surgical procedure. It involves a series of evaluations, procedures, and consultations before and after the actual operation, so people interested in being treated with DBS should be prepared to commit time to the process.
For example, those who do not live close to a medical center that offers DBS surgery may need to spend significant time traveling back and forth to appointments.
The procedure, as well as the pre-operative evaluation and post-operative follow-up, can be expensive depending on the persons insurance coverage. DBS surgery is an FDA-approved treatment for Parkinsons disease, and Medicare and most private insurers cover the procedure, but the extent of coverage will depend on each persons individual policy.;
Prospective patients should have realistic expectations about DBS results. Although DBS can improve movement symptoms of Parkinsons disease and greatly improve quality of life in properly selected patients, it is not likely to return anyone to perfect health.
Recommended Reading: Does Parkinson’s Run In Families
Quality Assessment And Data Extraction
The Newcastle-Ottawa Quality Assessment Scale was used to assess the methodological quality of the prospective or retrospective cohort studies. Three major components of each study were examined: patient selection; comparability of the intervention and the observation groups; and outcome assessment. Controversial items were discussed with the primary investigator before final consensus was reached. The final selected studies were independently assessed by two reviewers using a standardized form. Data on following variables were retrieved from these articles: name of first author, publication year, sample size, UPDRS scores, and DBS targets.
Pallidal Stimulation Versus Subthalamic Stimulation
As mentioned, there are two main anatomic targets for using DBS to treat PD the STN and the GPi. There have been several large randomized studies comparing STN and GPi DBS in PD. It is suggested that both STN DBS and GPi DBS overall equally and successfully improve motor symptom, and are similar in cost-effectiveness. ;However, although no differences were observed in the on phase between STN DBS and GPi DBS, significant differences were seen in the off phase; STN DBS was more effective in terms of motor function improvement in the off phase. There are different opinions in terms of effects of STN DBS and GPi DBS on quality of life. Some authors have found no significant difference between the STN and GPi targets. However, others agree with that greater improvements in quality-of-life measures are achieved in patients with GPi DBS.
GPi DBS can be used for patients with more axial symptoms, gait issues, dyskinesias, depression, and word fluency problems. STN DBS is often favored in reducing medication post surgery, and for patients with greater tremor. ;STN-DBS has also demonstrated an improvement in the quality of sleep for patients.
Also Check: Parkinson’s Personality Changes
Comparison Of Efficacy Of Deep Brain Stimulation Of Different Targets In Parkinson’s Disease: A Network Meta
- Department of Neurosurgery, Chinese PLA General Hospital, Beijing, China
Background: Deep brain stimulation is considered an effective treatment option for Parkinson’s disease . Several studies have demonstrated the efficacy of neurostimulation in patients with advanced PD. The subthalamic nucleus , the internal globus pallidus , ventral intermediate nucleus , and pedunculopontine nucleus are reportedly effective DBS targets for control of Parkinsonian tremors. However, there is no consensus on the ideal target for DBS in patients with Parkinson’s disease. Only a few studies have directly compared the efficacy of DBS of the Vim, STN, and GPi. Therefore, we searched PubMed, Embase, Cochrane Library, and other databases for observational studies, extracted data on unified Parkinson’s disease rating scale scores and performed a comprehensive network meta-analysis of different strategies of DBS and compared the efficiency of DBS at different targets.
Methods: Forest plot was used to examine the overall efficiency of DBS; cumulative probability value was used to rank the strategies under examination. A node-splitting model was employed to assess consistency of reported outcomes inconsistency. A total of 16 studies which focused on UPDRS improvement were included in the network meta-analysis.
Our findings will help improve clinical application of DBS.
After 15 Years Deep Brain Stimulation Still Effective In People With Parkinson’s
by American Academy of Neurology
Deep brain stimulation continues to be effective in people with Parkinson’s disease 15 years after the device is implanted, according to a study published in the June 2, 2021, online issue of Neurology.
Researchers found that compared to before deep brain stimulation, study participants continued to experience significant improvement in motor symptoms, which are symptoms that affect movement, as well as a reduction in medications 15 years later.
Parkinson’s disease can progressively affect speech, walking and balance due to a gradual reduction of a chemical in the brain called dopamine. Parkinson’s symptoms of muscle stiffness, tremor and slowness of movement can be treated with a medication call levodopa that temporarily restores dopamine. However, that process of rising and falling levels of dopamine throughout the day can cause dyskinesia, a side effect of medication that may include twisting, swaying or head bobbing.
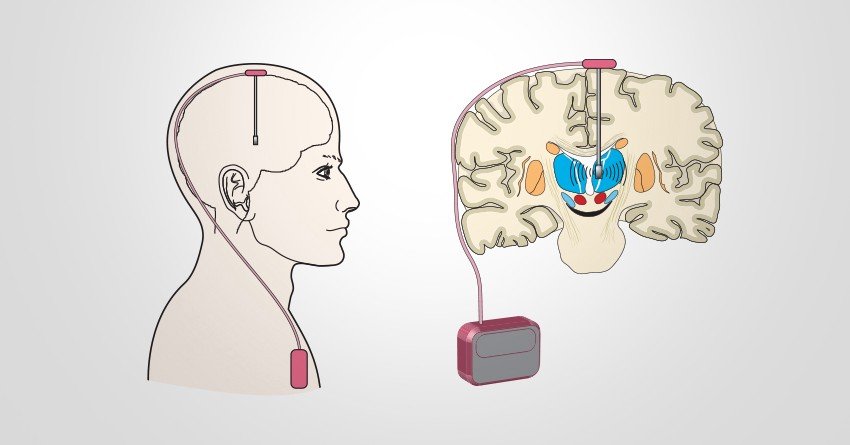
Deep brain stimulation controls motor symptoms from Parkinson’s disease with electrodes that are placed in certain areas of the brain. The electrodes are connected to a device placed under the skin in the upper chest. The device controls the electrical impulses.
Researchers reviewed data for each participant on movement problems, quality of life, medication and scores on tests that measure the severity and progression of Parkinson’s disease.
Explore further
You May Like: What Is The Life Expectancy For Someone With Parkinson Disease
Deep Brain Stimulation Dbs
DBS is already established as a treatment for patients with advanced Parkinsons disease.
It is not a cure, and it does not stop the disease from progressing, but in the right patients, it can significantly improve symptoms, especially tremors, and it can also relieve muscle rigidity.
To perform DBS, the neurosurgeon drills a hole in the skull and inserts an electrode about 10 cm into the brain. The electrode delivers mild electrical signals that disrupt and block the brain impulses that cause Parkinsons symptoms.
A wire under the skin connects the electrode to a battery implanted near the collarbone.
DBS can be done on one or both sides of the brain. The target areas are usually the thalamus, subthalamic nucleus, and globus pallidus. In this study, the target area was the thalamus.
Stereotactic Dbs Vs Interventional Image
Stereotactic DBS surgery requires the patient to be off their medication. During the procedure, a frame stabilizes the head and provides coordinates to help the surgeons guide the lead to the correct location in the brain. The patient gets local anesthesia to keep them comfortable throughout each step along with a mild sedative to help them relax.
During image-guided DBS surgery, such as with interventional MRI or CT scan, the patient is often asleep under general anesthesia while the surgeon uses images of the brain to guide the lead to its target.
Some advanced centers offer both the stereotactic and iMRI-guided options for DBS surgery. In this case, the doctor and patient will discuss which procedure is better based on a number of factors.
For instance, the doctor may recommend an image-guided procedure for children, patients who have extreme symptoms, those who are especially anxious or fearful or those whose leads are going into certain parts of the brain.
Generally, DBS surgery follows this process:
Also Check: Parkinson’s Sleep Disorder
Awake Vs Asleep Surgery
Standard DBS surgery is performed while you are awake and requires that you stop taking the medicines that control your Parkinson’s symptoms. During surgery, you are asked to perform tasks to help guide the electrode to the precise location in the brain.
Being awake during brain surgery, or being off medicine, is unsettling for some people. Asleep DBS is an alternative option at some centers.
Asleep DBS surgery is performed while you are unconscious and under anesthesia. Surgery takes place in an MRI or CT scanner to target and verify accurate placement of your DBS electrodes. Ask your surgeon if asleep DBS is an option for you.
| ;;; | |
| Must hold medications the morning of surgery | Don’t have to hold medications |
Deep Brain Stimulation Still Effective After 15 Years In People With Parkinson Disease Study Shows
Deep brain stimulation of the subthalamic nucleus was shown to remain effective in treating motor complications of people with Parkinson disease 15 years after initial surgery.
Deep brain stimulation of the subthalamic nucleus may prove effective after more than 15 years in patients with Parkinson disease , according to study findings published last week in Neurology.
Associated with several positive outcomes in patients with PD, including of disease progression, researchers say that STN-DBS has been shown to maintain efficacy in patients up to 11 years after surgery. However, conflicting reports have indicated DBS may lose efficacy over time.
Initial post-operative quality of life improvement has been described to fall to preoperative levels after 5-year stimulation, likely due to the escalation of both levodopa- and stimulation-resistant motor and nonmotor features of PD, such as impairments of gait, balance, speech and cognition, said the study authors.
With a growing rate of PD diagnoses and number of STN-DBS procedures conducted, researchers note that large data examining efficacy after the second and third decades post-procedure is lacking, with small populations having been the focus of previous studies examining motor response from STN-DBS after more than 10 years.
A mean follow-up time of 17.06 ± 2.18 years was reported among the study cohort.
Reference
You May Like: Is Parkinsons Fatal
Research To Improve Deep Brain Stimulation
Researchers are working to improve upon existing DBS devices and methods to;help treat more symptoms and more people. Some researchers are putting electrodes in a different area of the brain the pedunculopontine nucleus to treat walking and balance problems that don’t typically improve with present-day DBS. Others are developing a “smart” DBS device that can record a person’s unique brain signals and deliver electrical stimulation only when needed, such as when symptoms return, rather than continuously, as the current systems do. This could help reduce side effects such as numbness and weakness and lengthen the battery life of the neurostimulator, which would result in a longer time between battery replacement procedures.
Scientists also are planning to test;deep brain stimulation in the first years after a Parkinson’s diagnosis to see if the therapy may slow or stop disease progression. Testing in Parkinson’s models showed the therapy may help protect brain cells, and a small human trial showed motor symptoms improved after early-stage DBS.
