Mitochondria And Calcium Homeostasis
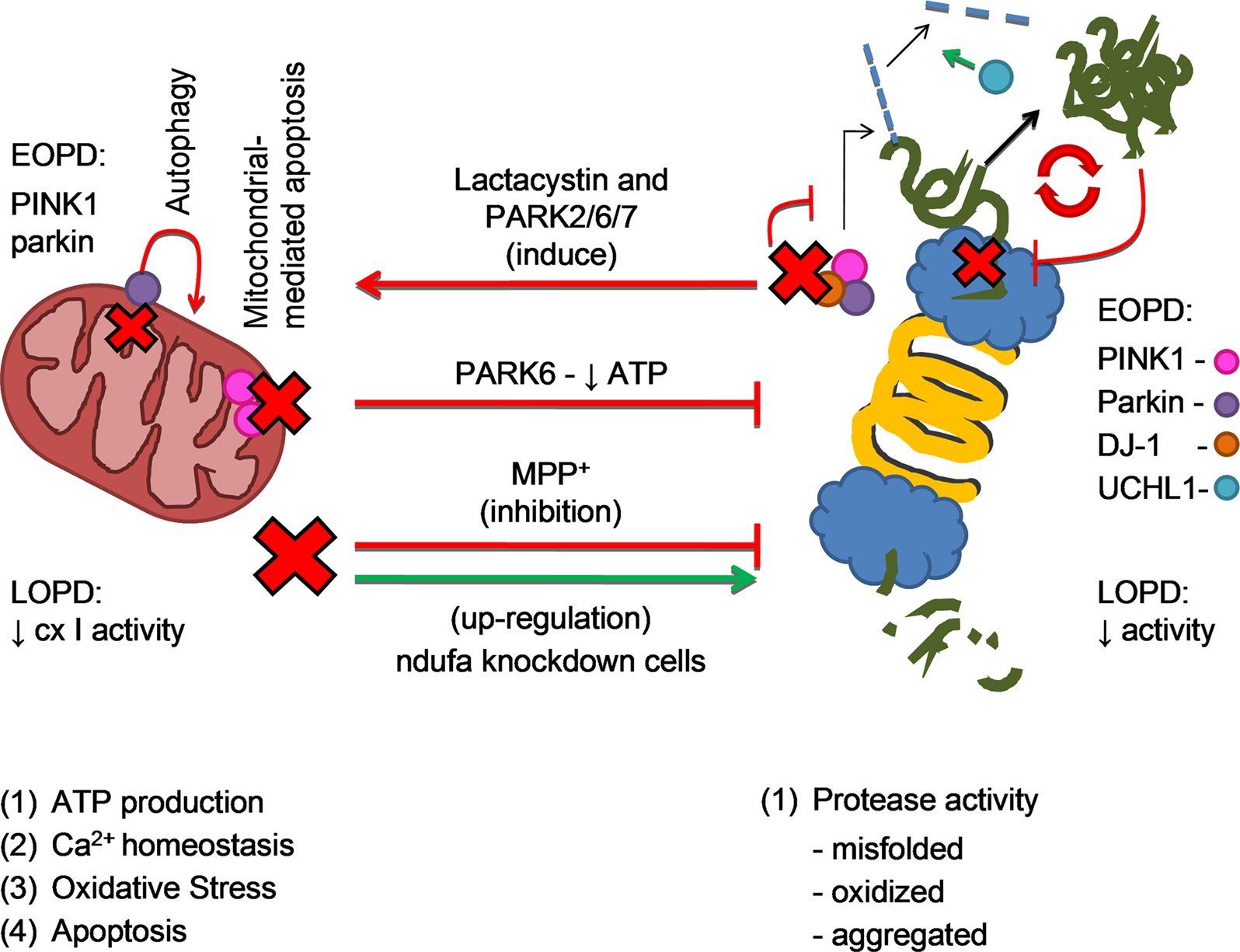
Other studies further support a role for alterations in mitochondrial Ca2+ homeostasis in PD. For instance, cybrid cells containing mtDNA from PD patients exhibit lower mitochondrial Ca2+ sequestration than control cells following carbachol-stimulated Ca2+ entry . Similarly, parkinsonian neurotoxins MPP+ and rotenone cause diminished mitochondrial Ca2+ uptake and increased cytosolic free Ca2+ in cultured cells . Also, exogenously applied oligomeric, but not monomeric, -synuclein to cultured dopaminergic neurons was shown to increase intracellular Ca2+ levels through a pore-mediated influx of extracellular Ca2+, leading to increased mitochondrial Ca2+-buffering burden and apoptotic cell death . Furthermore, PD-related protein PINK1 appears to regulate the physiological release of Ca2+ from the mitochondria via the mitochondrial Na+/Ca2+ exchanger . Indeed, ablation of PINK1 in dopaminergic neurons leads to impaired Ca2+ efflux from mitochondria, accumulation of mitochondrial Ca2+, increased production of mitochondrial ROS, decreased mitochondrial respiration, reduced , and a lowered threshold for Ca2+-dependent opening of the mitochondrial permeability transition pore complex, overall resulting in increased apoptosis . Supporting a role for increased Ca2+ load in PD-related cell death in vivo, pharmacological or genetic inhibition of Ca2+-sensitive proteases has been shown to attenuate dopaminergic neurodegeneration in MPTP-intoxicated mice .
The Molecular Basis For An
In contrast to its intrinsically disordered state in solution, -Syn adopts a highly helical conformation when associated with lipids . When any of the three exons encoding its N-terminal domain are deleted, the interaction between -Syn and vesicles is disrupted . These deletions also reduce the presynaptic localization of -Syn, suggesting that its reduced association with vesicles also impairs its transport along axons . Further proof that the N-terminus of -Syn associates with membranes comes from the protection of its residues 1 to 103 from proteolytic cleavage when -Syn is incubated with sodium dodecyl sulfate micelles or with lipid vesicles .
In in vitro studies, the binding of -Syn to large unilamellar vesicles correlates with the concentration of the non-bilayer-forming lipid cardiolipin , a type of lipid enriched in mitochondrial membranes . Another study has shown that the binding of -Syn to intact mitochondria can be reduced using nonyl acridine orange , and that stripping external proteins from mitochondria has no effect on the association between -Syn and mitochondria . This suggests that this association is not protein dependent . The protonation of -Syn under acidic intracellular pH also increases its binding to mitochondrial membranes, and this effect can be explained by the high content of acidic phospholipids in mitochondria .
Are Alterations In The Axonal And Synaptic Mitochondrial Populations Correlated With Respiratory Deficiency In The Soma
Although in human tissue it is not possible to compare cell body changes to synaptic/axonal changes within the same neuron, a measure of respiratory chain deficiency was made within the soma of SN neurons for most of the cases included in this study . Intensity measurements were made for immunoreactivity for NDUFB8, COXI and porin within TH positive cell bodies within the SN. A reduction in expression of either of these proteins was defined as an intensity value that had a z score of less than 1, normal neurons had a score of between 1 and 1, while an increase was defined as having a score over 1. These parameters were set based on the control data.
There was an increase in the percentage of cell bodies showing reduced expression of both complex I and complex IV in PD cases compared to other groups, and in DLB cases there was an increase in complex IV deficient neurons, however neither of these changes was significant due to the variability between cases . In addition to measuring deficiency within the neurons of the SN, mitochondrial density was also quantified. Using the signal intensity for porin immunoreactivity we categorised each neuron based on the z score for porin. We again found that there was no significant difference between the mitochondrial mass in cell bodies between any of our patient groups .
You May Like: Is Gabapentin Used For Parkinson’s Disease
Ermitochondria Signaling In Neurodegeneration
Neurodegenerative diseases including PD, AD, and ALS/FTD share several obvious features: they are characterized by progressive nervous system dysfunction, affect millions of people worldwide and there is still no cure for any of them. Furthermore, despite affecting different brain regions PD, AD, and ALS/FTD also share other characteristics suggesting that common cellular processes may converge.
Thus, whilst the precise mechanisms remain to be determined, a variety of cellular processes are damaged in all of them, including Ca2+ dysregulation, defects in axonal transport, neuroinflammation, loss of cellular proteostasis and mitochondrial dysfunction,,,,, . Remarkably, ERmitochondria associations, regulates all of those processes. The findings that alterations in ERmitochondria associations occur in neurodegenerative diseases have given rise to the hypothesis that damaged ERmitochondria signaling is a common potential therapeutic target amongst distinct age-dependent neurodegenerative disorders.
Fig. 3: Proposed model for endoplasmic reticulummitochondria signaling in PD
ERmitochondrial axis appears to be essential for the healthy neurons. Conversely, the disruption of this interaction may involve the develop of some processes as: mitochondrial dysfunction, induction of oxidative stress, calcium dyshomeostasis, autophagy defects or neuroinflammation, which induce neuronal damage and trigger neurodegenerative diseases as PD
-Synuclein
PINK1 and Parkin
DJ-1
Diabetes A Contemporary Risk For Parkinsons Disease: Epidemiological And Cellular Evidences
- 1Nutrition and Health Substantiation Group, Nutrition and Health Program, Health and Biosecurity, Commonwealth Scientific and Industrial Research Organisation , Adelaide, SA, Australia
- 2Adelaide Medical School, University of Adelaide, Adelaide, SA, Australia
- 3Cellular Neurobiology, Department of Medical Biology, Université du Québec, Trois-Rivières, QC, Canada
- 4Department of Biomedical Sciences, University of Cagliari, Cagliari, Italy
- 5National Institute for Neuroscience , University of Cagliari, Cagliari, Italy
- 6Department of Psychiatry and Neuroscience, Université Laval and CHU Research Center, Québec, QC, Canada
Read Also: Can Parkinson’s Disease Cause Back Pain
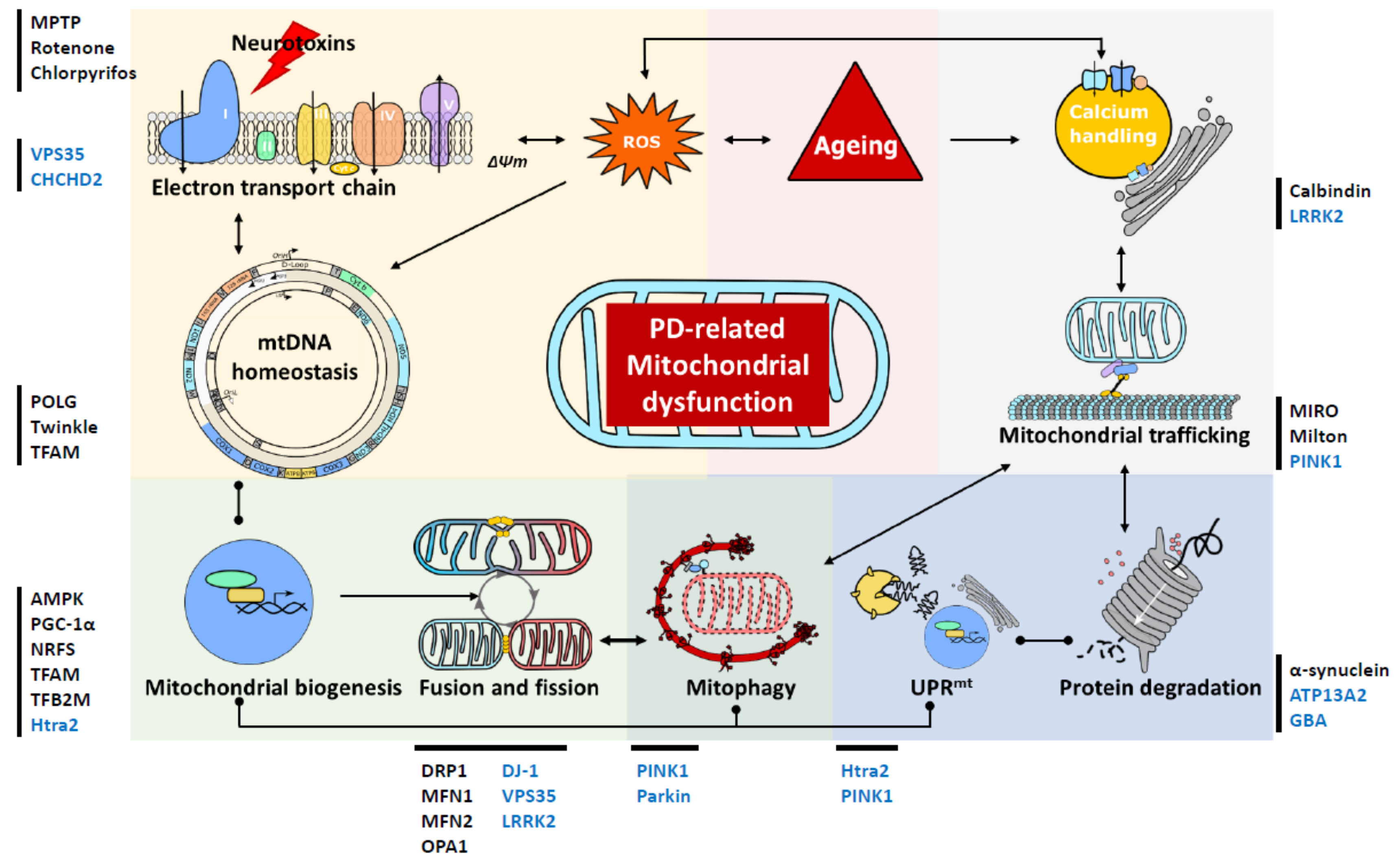
Restoring Mitochondrial Dynamics And Trafficking
Mitochondria form a complex network, the cohesiveness and shape of which is of direct functional relevance . Mitochondria are highly dynamic, constantly breaking off, spatially relocating, and rejoining the network. This is critical for neurons as their complex architecture requires mitochondrial relocation from the soma to dendrites, axons and synapses to meet regional metabolic demands . The majority of genetic PD proteins locating to mitochondria and mitochondria-ER contact sites are associated with processes influencing or influenced by mitochondrial dynamics and trafficking . A comprehensive summary of mitochondrial dynamics and trafficking in neuronal function can be found elsewhere .
Mitochondrial dynamics are highly complex but strictly balanced processes controlled by a diverse array of established and emerging molecular mediators, in addition to mitochondria-ER contact sites . Fragmentation of the mitochondrial network is a salient observation in PD, highlighting mitochondrial dynamics as a key therapeutic target. To this end, mdivi-1, an inhibitor of the mitochondrial fission GTPase Drp1, has been used to inhibit mitochondrial fragmentation in an -synuclein rat model of PD, reducing neurodegeneration, -synuclein aggregation, mitochondrial dysfunction and oxidative stress . While therapeutic manipulation of mitochondrial dynamics shows great promise, it will require greater understanding to be effectively exploited.
What Genes Are Linked To Parkinsons Disease
Several genes have been definitively linked to PD:
- SNCA. This gene, which makes the protein alpha-synuclein, was the first gene identified to be associated with Parkinsons. Research findings by the National Institutes of Health and other institutions prompted studies of the role of alpha-synuclein in PD, which led to the discovery that Lewy bodies seen in all cases of PD contain clumps of alpha-synuclein. This discovery revealed the link between hereditary and sporadic forms of the disease.
- LRRK2. Mutations in LRRK2 were originally identified in several English and Basque families as a cause of a late-onset PD. Subsequent studies have identified mutations of this gene in other families with PD as well as in a small percentage of people with apparently sporadic PD. LRRK2 mutations are a major cause of PD in North Africa and the Middle East.
- DJ-1. This gene normally helps regulate gene activity and protect cells from oxidative stress and can cause rare, early forms of PD.
- PRKN . The parkin gene is translated into a protein that normally helps cells break down and recycle proteins.
- PINK1. PINK1 codes for a protein active in mitochondria. Mutations in this gene appear to increase susceptibility to cellular stress. PINK1 has been linked to early forms of PD.
- GBA . Mutations in GBA cause Gaucher disease , but different changes in this gene are associated with an increased risk for Parkinsons disease as well.
Also Check: What Percent Of Population Has Parkinson’s
How Can People Cope With Parkinson’s Disease
While PD usually progresses slowly, eventually daily routines may be affectedfrom socializing with friends to earning a living and taking care of a home. These changes can be difficult to accept. Support groups can help people cope with the diseases emotional impact. These groups also can provide valuable information, advice, and experience to help people with PD, their families, and their caregivers deal with a wide range of issues, including locating doctors familiar with the disease and coping with physical limitations. A list of national organizations that can help people locate support groups in their communities appears at the end of this information. Individual or family counseling may also help people find ways to cope with PD.
People with PD may also benefit from being proactive and finding out as much as possible about the disease in order to alleviate fear of the unknown and to take a positive role in maintaining their health. Many people with PD continue to work either full- or part-time, although they may need to adjust their schedule and working environment to accommodate their symptoms.
Do Symptoms Get Worse
PD does not affect everyone the same way. The rate of progression and the particular symptoms differ among individuals.
PD symptoms typically begin on one side of the body. However, the disease eventually affects both sides, although symptoms are often less severe on one side than on the other.
Early symptoms of PD may be subtle and occur gradually. Affected people may feel mild tremors or have difficulty getting out of a chair. Activities may take longer to complete than in the past. Muscles stiffen and movement may be slower. The persons face may lack expression and animation . People may notice that they speak too softly or with hesitation, or that their handwriting is slow and looks cramped or small. This very early period may last a long time before the more classical and obvious motor symptoms appear.
As the disease progresses, symptoms may begin to interfere with daily activities. Affected individuals may not be able to hold utensils steady or they may find that the shaking makes reading a newspaper difficult.
People with PD often develop a so-called parkinsonian gait that includes a tendency to lean forward, taking small quick steps as if hurrying , and reduced swinging in one or both arms. They may have trouble initiating movement , and they may stop suddenly as they walk .
Don’t Miss: What Does A Neurologist Do For Parkinson’s
Microglial Activation And Systemic Chronic Inflammation Increase The Risk Of T2dm And Pd
Microglia are mononuclear, phagocytic immune cells in the central nervous system. They are normally involved in removal of damaged neurons, and they release neuroprotective factors to promote synaptic regeneration . Microglia can be activated towards either an anti-inflammatory or inflammatory phenotype. For example, microglia can be stimulated by lipopolysaccharide to enter an activated inflammatory state and express pro-inflammatory cytokines such as TNF?, interleukin 1? and IL6, triggering neuroinflammation . A study of 14 patients with PD found evidence of increased microglial activation on PET imaging . Microglial activation is a key contributor to neuroinflammation through the release of inflammatory cytokines , and patients with PD have been shown to have high concentrations of inflammatory mediators such as IL1?, IL6 and TNF? in the brain .
What Diseases And Conditions Resemble Parkinsons Disease
PD is the most common form of parkinsonism, in which disorders of other causes produce features and symptoms that closely resemble Parkinsons disease. Many disorders can cause symptoms similar to those of PD, including:
Several diseases, including MSA, CBD, and PSP, are sometimes referred to as Parkinsons-plus diseases because they have the symptoms of PD plus additional features.
In very rare cases, parkinsonian symptoms may appear in people before the age of 20. This condition is called juvenile parkinsonism. It often begins with dystonia and bradykinesia, and the symptoms often improve with levodopa medication.
You May Like: Can Heart Surgery Cause Parkinson’s
What Is Parkinson’s Disease
Parkinsons disease is movement disorder of the nervous system that worsens over time. As nerve cells in parts of the brain weaken or are damaged or die, people may begin to notice problems with movement, tremor, stiffness in the limbs or the trunk of the body, or impaired balance. As these symptoms become more obvious, people may have difficulty walking, talking, or completing other simple tasks. Not everyone with one or more of these symptoms has PD, as the symptoms appear in other diseases as well.
No cure for PD exists today, but research is ongoing and medications or surgery can often provide substantial improvement with motor symptoms.
Mitochondrial Dysfunction And Parkinsons Diseasenear
- 1Department of Physiology, National University of Singapore, Singapore, Singapore
- 2Division of Neurosurgery, Department of Surgery, University Surgical Cluster, National University Hospital, Singapore, Singapore
- 3Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore
- 4Department of Research, National Neuroscience Institute, Singapore, Singapore
Read Also: Is Parkinson’s A Genetic Disorder
Earlier Research On The Link Between Type 2 Diabetes And Parkinsons Disease
Some previous research has linked certain medications for type 2 diabetes to a lower risk of the development or progression of Parkinsons disease.
A found Parkinsons disease symptoms improved in participants who took exenatide, a diabetes drug in a family of medicines known as GLP1 agonists, and worsened when subjects took a placebo. Another , found that individuals with type 2 diabetes who took GLP1 agonists or another type of diabetes drugs known as DPP4 inhibitors had a lower risk of developing Parkinsons disease.
Slightly elevated blood sugar or variations in blood sugar may contribute to the risk of Parkinsons disease even in people without diabetes, according to a .
Age is the biggest risk factor for Parkinsons disease, though, and genetics also account for up to 20 percent of the risk, Foltynie says.
RELATED: How to Keep Your Brain Healthy: A Conversation With Sanjay Gupta, MD
Mitochondrial Fusion And Fission
The hundreds of mitochondria within a cell undergo continual cycles of fusion and fission , resulting in a wide range of mitochondrial morphologies . The adequate balance between fusion and fission is crucial for the maintenance of mitochondrial function. For instance, mitochondrial fusion is required for the proper respiratory activity of the mitochondria and has been associated with cell survival . In addition, the functionality of damaged mitochondria can be restored by exchanging mitochondrial genomes and gene products by fusion with neighboring, intact mitochondria, thereby attenuating the potential deleterious effects of misfolded proteins or mutated mtDNAs . The proper localization of mitochondria to nerve terminals also depends on the correct balance between mitochondrial fusion and fission, as fragmentation of the mitochondrial network by fission appears to facilitate the recruitment of mitochondria to nerve terminals .
You May Like: Can Parkinson’s Dementia Be Reversed
Linking Mitochondrial Dysfunction With Pd
The mitochondrial theory of PD is based on observations in which disease processes impairing oxidative phosphorylation, mitochondrial biogenesis or mitophagy manifest as phenotypes that share common Parkinsonian features. The first link between PD and mitochondrial dysfunction was conceived by Langston after observing rapidly developing Parkinsonian features in intravenous drug abusers who were exposed to 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine , a toxin whose metabolite 1-methyl-4-phenylpyridinium inhibits mitochondrial complex I . Once in the central nervous system, MPP+ is taken up by the dopamine active transporter and induces the selective degeneration of SNpc dopaminergic neurons. This phenomenon has been successfully reproduced in MPTP-treated rodents and nonhuman primates, popularizing these animal models for PD research . Complementing the findings in MPTP, rotenone, a pesticide that inhibits mitochondrial complex I, produced similar PD-like locomotor deficits in treated animals. Despite it having widespread uptake in the central nervous system, the toxic effects of rotenone are most pronounced in SNpc dopaminergic neurons, reinforcing the belief that neurons with greater susceptibility to energy perturbances would be affected the most . Further evidence is gleaned from epidemiological studies, which identified rotenone-exposed agrarian populations to be at greater risk of developing PD .
Origins Of The Link Between Mitochondria And Pd
First, the so-called frozen addicts suggested a contribution of mitochondrial dysfunction to the pathogenesis of PD. In these drug users, living in California in the 1980s, physicians observed that a side product of new synthetic heroin triggered a rapid onset of a distinct form of parkinsonism responsive to levodopa treatment. It turned out that the synthesis process resulted in the unwanted generation of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine , which led to inhibition of the respiratory chain . Of note, a similar observation was published already four years earlier . MPTP is not toxic itself but lipophilic and thus able to enter brain tissue by crossing the blood brain barrier. In the brain, it is processed by monoamine oxidase B to the toxic cation 1-methyl-4-phenylpyridinium . MPP+is selectively taken up by dopaminergic cells and inhibits multiple complexes of the respiratory chain . The notion that mitochondrial dysfunction plays a role in PD pathogenesis was supported shortly after the description of the frozen addicts by the observation of a restricted function of respiratory chain complexes in postmortem brain sections from PD patients . These early findings significantly stimulated PD research in the following years. For example, even today, the injection of MPTP is most commonly used to model PD in mice . However, similar to other animal models of PD, the clinical and pathological characteristics simulated by the MPTP model differ from PD in many ways .
Recommended Reading: Are Bananas Good For Parkinson’s
The Mitochondrial Oxidative Phosphorylation System
Mitochondria are usually considered the powerhouses of the cell because of the production of ATP, via the combined efforts of the tricarboxylic acid cycle and the respiratory chain/oxidative phosphorylation system. The respiratory chain, located in the IMM, consists of five multimeric protein complexes: reduced nicotinamide adenine dinucleotide dehydrogenase-ubiquinone oxidoreductase , succinate dehydrogenase-ubiquinone oxidoreductase , ubiquinone-cytochrome c oxidoreductase , cytochrome c oxidase , and ATP synthase . The respiratory chain also requires two small electron carriers, ubiquinone/coenzyme Q and cytochrome c. ATP synthesis involves two coordinated processes: electrons derived from energy substrates are transported through the different mitochondrial complexes to molecular oxygen, thereby producing water; at the same time, protons are pumped across the mitochondrial inner membrane by complexes I, III, and IV, generating an electrochemical gradient . ATP is produced by the influx of these protons back into the mitochondrial matrix through complex V . ATP is the main form of energy used by the cell and, once produced in the mitochondrion, it is exported to the cytosol by the adenine nucleotide translocator in exchange for cytosolic ADP.
