Mitochondrial Dysfunction In Sporadic Parkinsons Disease
Sporadic PD occurs as a seemingly random occurrence due to undetermined genetic or environmental bases in the absence of an obvious family history. It is well established that PD is a multifactorial disorder caused by impaired cellular functions that impact upon interrelated pathways and create complex feedback cycles leading to neurodegeneration . Broadly, affected cellular pathways include proteostasis, oxidative stress and the multiple pathways relating to mitochondrial function 1) , all of which are evident in sporadic PD.
Animals And Stereotaxic Surgery
Adult male and female Lewis rats were obtained from Envigo . Rats were singly housed in temperature-controlled conditions under a 12:12 light-dark cycle with ad libitum access to food and water. All experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Stereotaxic surgery was performed to deliver virus to the substantia nigra . Animals were deeply anesthetized under isoflurane for the entirety of the surgery. Two microliter of virus was infused over a 10-min period . Animals were closely monitored on a heated surface during recovery of surgery. Animals received analgesia in the form of ketoprofen with the first dose presurgery and 2Ã/day for 3 days following surgery. Animals were sacrificed by transcardial perfusion with phosphate buffered saline followed by 4% paraformaldehyde. The brains were harvested and sectioning was performed on a freezing-stage microtome at 35µm. Sections were maintained in cryoprotectant at 20°C until analysis.
How To Determine The Dopamine Level In The Brain Of Parkinsons Patients
There is no rigorous method that could directly access to Dopamine and monitor its changes in the brain. The currently used methods rely on brain imaging techniques which do not reliably detect and measure Dopamine changes in the human brain.
Recently, neuroscientists at the Massachusetts Institute of Technology, Cambridge have developed a method to measure Dopamine in the brain for a long period of time, more than a year. They have designed a sensor which they believe could be used to monitor the changes in Dopamine levels in brain areas where Dopamine is highly concentrated. The sensor is so small that it can be implanted in different parts of the brain. The sensor has been successfully tested on animals and hopefully will be available for human trials in the near future.
Also Check: Is Beer Good For Parkinson’s
Multiple Mitochondria Functions: Evidences From Pink1/parkin And Other Pd
It is noteworthy that a more extensive insight into the autosomal recessive models of PD, particularly PINK1– and Parkin-associated PD, revealed a wider role of mitochondria in PD pathogenesis, beyond the view of a solely ATP- and ROS-producing organelle . The autosomal recessive models uncovered the importance of multiple mechanisms that maintain healthy mitochondrial pool and then neurons. These mitochondrial pathways are represented by mitochondrial quality control mechanisms, especially mitophagy, mitochondrial trafficking, and mitochondrial calcium homeostasis maintenance. In the next paragraphs, we will try to explain the relationship between PD and these more recently discovered mitochondrial pathways.
Figure 1. The complex role of PINK1/Parkin pathway in mitochondria. MDVs, mitochondrial-derived vesicles; QC, quality control; PARIS, Parkin interacting substrate; PGC-1alpha, peroxisome proliferator-activated receptor-gamma coactivator; UPRmt, mitochondrial unfolded protein response.
Living With Parkinsons Disease
As Parkinsonâs develops, a person who has it may slow down and wonât be able to move or talk quickly. Sometimes, speech therapy and occupational therapy are needed. This may sound silly, but someone who has Parkinsonâs disease may need to learn how to fall down safely.
If getting dressed is hard for a person with Parkinsonâs, clothing with Velcro and elastic can be easier to use than buttons and zippers. The person also might need to have railings installed around the house to prevent falls.
If you know someone who has Parkinsonâs disease, you can help by being a good friend.
Don’t Miss: What Medications Should Parkinson’s Patients Avoid
Common Pathogenic Mechanisms Of Systemic And Brain Insulin Resistance
As epidemiological evidence for a link between PD and T2DM accumulates, parallel experimental evidence indicates potential overlap in disease mechanisms and pathways. Systemic insulin resistance has long been an established key feature of T2DM. Recently, studies have found that insulin resistance is present in the brain in neurodegenerative diseases such as Alzheimers disease and other dementias , and PD . Both systemic and local insulin resistance may drive pathology in the brain. Systemic insulin resistance may do so through hyperglycaemia and its consequences , microvascular disease, chronic inflammation, and dysfunction of the blood brain barrier, which may be compounded by associated comorbidities such as hypertension, dyslipidaemia and renal impairment . Local brain insulin resistance may act via protein deposition and aggregation, and failure of clearance mechanisms, independent of systemic insulin resistance .
Crosstalk Between Mitochondria Lysosomes And Er And Its Impact On Calcium Homeostasis
Multiple lines of evidence suggest that impaired lysosomal degradation causes an accumulation of dysfunctional mitochondria in PD . Mutations in LRRK2 and SNCA have been demonstrated to interfere with lysosomal pathways. Furthermore, in DJ-1-mutant iPSC-derived neurons, mitochondrial stress was shown to trigger oxidized dopamine accumulation, which in turn led to lysosomal dysfunction, and eventually the accumulation of alpha-synuclein .
In addition to the crosstalk between lysosomes and mitochondria, the ER is involved in the inter-organellar communication in PD. Alterations of the MAM have been described in different PD models . Exemplarily, alpha-synuclein can be found at the MAM, and pathogenic mutations in SNCA lead to increased mitochondrial fragmentation .
Furthermore, calcium homeostasis depends on a well-orchestrated signalling between mitochondria, the lysosome and the ER. In SNCA overexpression models and patient-derived neurons with a triplication mutation, a reduced connection between ER and mitochondria leads to a calcium-dependent decrease in ATP production . However, also Parkin , PINK1 and LRRK2 , as well as DJ-1 may function in calcium-related pathways.
Emphasizing the role of calcium homeostasis in PD, research demonstrated that isradipine, a calcium channel antagonist, protects dopaminergic neurons by lowering mitochondrial oxidative stress and by reducing mitochondrial turn over and mass .
You May Like: How Does Deep Brain Stimulation Work In Parkinson’s Disease
Changes In Synaptic Volume In Parkinsons Disease
To ascertain whether any structural changes occurred within synapses of substantia nigra neurons we measured the volume of both pre-synaptic terminals and their corresponding post-synaptic region. We employed dual immunofluorescence for the dopamine transporter and the dopamine D2 receptor and the number of immunoreactive objects per image was then calculated. We detected, as expected, a significant reduction in pre-synaptic DAT positive terminals within the putamen in PD and dementia with Lewy bodies cases, compared to controls and AD cases . Interestingly we also detected a loss of D2R positive, post synaptic terminals in PD and AD cases compared to controls and in PD cases compared to DLB cases .
Fig. 1
Representative images of pre and post synaptic immunoreactivity. Synaptic terminals of dopaminergic SN neurons within the striatum were analysed. Pre-synaptic terminals were visualised using reactivity for the dopamine transporter, DAT . Images were taken of pre-synaptic terminals surrounding a post synaptic neuron, post synaptic regions were visualised using reactivity for the dopamine D2 receptor . Pre and post synaptic volume was measured in tissue from patients with PD , DLB , AD and in age matched controls . Scale bar represents 10µm
Dopaminergic Input And Organizational Features Of The Dorsal And Lateral Striatum
As reviewed above, it is generally accepted that dysfunction in PD stems from the degeneration of SNc neurons , which leads to motor dysfunction and the loss of VTA neurons , which leads to behavioral dysregulation, including demotivation, anhedonia, and depression within PD . While both pathways have been studied extensively across an array of conditions and pathologies, the modulatory mechanisms of the nigrostriatal pathway neurons have been fairly well described while the varied mechanisms and roles of VTA efferents continue to be elucidated. Within the nigrostriatal pathway, GABAergic medium spiny neurons of the dorsal/lateral striatum receive excitatory glutamatergic signals that can be modulated via dopaminergic inputs originating from the SNc. MSNs are moderately sized cells with large, multi-structured dendritic arbors that constitute a staggering 95% of all postsynaptic nigrostriatal neurons . Local circuit interneurons of the dorsal striatum are also actively involved in regulating MSN activity and can be subdivided into cholinergic interneurons and aspiny GABAergic interneurons known as low-threshold, fast-spiking neurons . Striatal cholinergic and MSNs express several neurotransmitter receptors including the ?-aminobutyric acid , glutamate, DA, adenosine, serotonin, opioids, and substance P receptors .
Read Also: What Time Of Day Are Parkinson’s Symptoms Worse
Mitochondriathe Energy Powerhouses Of The Cell
The fact that energy-intensive neurons have increased vulnerability in PD suggests that some of the underlying disease processes may be linked to impairments in cellular energy production. This lends further weight to the hypothesis that disruptions in mitochondrial function constitute some of the key pathogenic processes in PD.
In addition to the regulation of oxidative phosphorylation, maintaining healthy mitochondrial volumes is also essential for efficient energy production in cells. The intracellular mitochondrial network is constantly pruned by the processes of biogenesis, fusion, fission, and degradation. These processes constitute the mitochondrial life cycle, which takes place every 520 min . Under physiological conditions, the events in the mitochondrial life cycle are in dynamic equilibrium, maintaining a stable mass of intracellular mitochondria. If cellular metabolic requirements are altered, the mitochondrial network undergoes remodeling to accommodate the changes in energy demand . The various stages in the mitochondrial life cycle are illustrated in Figure 1. Excellent reviews on the various aspects of the mitochondrial life cycle have been written elsewhere and we will therefore not elaborate on this topic in the present article .
Mitochondrial Dysfunction In Familial Parkinsons Disease
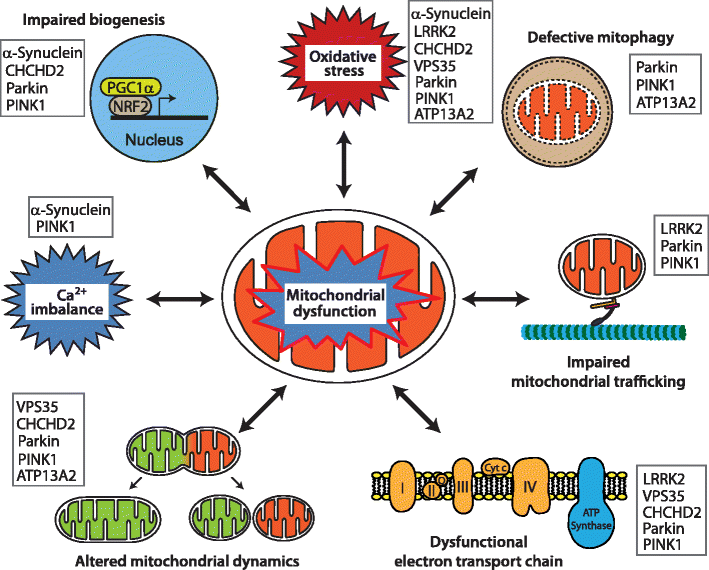
To date, a handful of genes have been identified as monogenic causes of familial PD, with many of the pathogenic mutations in these genes directly linked to mitochondrial dysfunction . More recently, new roles in the regulation of mitochondrial biology have been determined for these genes, and new PD genes associated with mitochondrial function, such as VPS35 and CHCHD2, have been identified, further underpinning the essential role of mitochondrial function to the aetiology of PD .
Representative pathways of mitochondrial dysfunction involved in Parkinsons disease pathophysiology. Mitochondrial dysfunction associated with PD pathogenesis can result from impairment of mitochondrial biogenesis, increased reactive oxygen species production, defective mitophagy, compromised trafficking, electron transport chain dysfunction, variations to mitochondrial dynamics, calcium imbalance or combinations thereof. The potential complex interplay of the various functions leads to a vicious cycle of progressive cellular dysfunction that ultimately results in neurodegeneration that underlies PD pathogenesis and progression. Proteins mentioned in this review that contribute pathologically to the different pathways are listed
You May Like: Why Is Parkinson’s On The Rise
Lrrk2 Ko Macrophages Exhibit Blunted Type I Ifn Induction In Response To Cytosolic Nucleic Acid Agonists
Lrrk2 KO macrophages exhibit blunted type I IFN expression in response to cytosolic nucleic acid agonists.
We next tested whether loss of LRRK2 impacted the ability of cells to respond to activators of the type I IFN response outside of the cytosolic DNA sensing cascade. To this end, we treated Lrrk2 KO and HET BMDMs with transfected poly , LPS , and CpG and CL097 . Interestingly, while we observed a defect in Ifnb induction in Lrrk2 KO BMDMs stimulated with poly, we saw no difference in the ability of Lrrk2 KO BMDMs to express type I IFNs following treatment with LPS, CL097, or CpG , suggesting that TLR responses are intact in the absence of LRRK2 but cytosolic DNA and RNA sensing pathways are perturbed. WeÂ;observedÂ;similar phenotypes for PEMs and MEFs treated with LPS and poly . Lrrk2 KO BMDMs were,Â;however,Â;defective in ISG expression following recombinant bioactive IFN- treatment .
Mitochondrial Dysfunction In Atypical Parkinsonisms
The role of mitochondrial dysfunction in the pathogenesis of atypical parkinsonism has been recently investigated. The involvement of mitochondria in pathogenetic pathways is demonstrated in both synucleinopathies and tauopathies. Multiple system atrophy , a synucleinopathy together with PD and dementia with Lewy bodies, is a neurodegenerative disorder in which a variable degree of parkinsonism, cerebellar ataxia, dysautonomia, and pyramidal features coexist. Considering the predominant symptomatology, parkinsonian or cerebellar subtype has been described . In neuropathological studies, MSA shows an accumulation of -syn in neurons and oligodendrocytes . Although the aberrant protein localization is known for many years, pathogenic mechanisms are almost unclear, and several processes such as -syn overexpression, cell-to-cell transfer, inflammation, and mitochondrial functioning have been proposed . A reduced complex I activity in MSA patients skeletal muscle , but not in platelets or SN , has been observed. Additionally, an impairment of enzymatic activities related to respiratory chain complex II in fibroblast primary cultures of MSA patients has also been demonstrated .
Read Also: How Does Age Affect Parkinson’s Disease
Parkinsons Disease: Why Dopamine Replacement Therapy Has A Paradoxical Effect On Cognition
- Date:
- University of Montreal
- Summary:
- Dopamine replacement therapy, which is used to manage motor symptoms associated with Parkinsons disease, can, at times, adversely affect cognition. Now researchers have identified the reasons why.
Dopamine replacement therapy, which is used to manage motor symptoms associated with Parkinsons disease, can, at times, adversely affect cognition. Dr. Oury Monchi, Ph. D. in neuronal modeling and Head of the Neurophysiological and Neuroimaging Research theme at the Centre de recherche de lInstitut universitaire de gériatrie de Montréal , which is affiliated with the Université de Montréal, and Dr. Penny A. MacDonald, Neurologist and postdoctoral fellow in Dr. Monchis laboratory, have identified the reasons why within the framework of a clinical study recently published in Brain: A Journal of Neurology.
Until now, the effect of dopamine replacement therapy on cognition in individuals with Parkinsons disease was controversial. The purpose of this study however, was to further investigate. This led to a series of laboratory tests and neuroimaging studies that allowed researchers to clearly define the distinct cognitive functions performed by the dorsal and ventral striatum, thereby shedding some light on the issue.
Summary of the Research
Parkinsons disease
The authors are grateful for the support provided by the IUGM Foundation and the Canadian Institutes of Health Research.
Story Source:
Understanding The Multiple Role Of Mitochondria In Parkinsons Disease And Related Disorders: Lesson From Genetics And Proteininteraction Network
- Unit of Neurology, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
As neurons are highly energy-demanding cell, increasing evidence suggests that mitochondria play a large role in several age-related neurodegenerative diseases. Synaptic damage and mitochondrial dysfunction have been associated with early events in the pathogenesis of major neurodegenerative diseases, including Parkinsons disease, atypical parkinsonisms, and Huntington disease. Disruption of mitochondrial structure and dynamic is linked to increased levels of reactive oxygen species production, abnormal intracellular calcium levels, and reduced mitochondrial ATP production. However, recent research has uncovered a much more complex involvement of mitochondria in such disorders than has previously been appreciated, and a remarkable number of genes and proteins that contribute to the neurodegeneration cascade interact with mitochondria or affect mitochondrial function. In this review, we aim to summarize and discuss the deep interconnections between mitochondrial dysfunction and basal ganglia disorders, with an emphasis into the molecular triggers to the disease process. Understanding the regulation of mitochondrial pathways may be beneficial in finding pharmacological or non-pharmacological interventions to delay the onset of neurodegenerative diseases.
Don’t Miss: Does Parkinson’s Affect Your Face
Increased Basal Type I Ifn In Lrrk2 Ko Macrophages Is Dependent On Cytosolic Dna Sensing Through Cgas
Because both IFN- blockade and loss of Ifnar normalized basal ISG expression in Lrrk2 KO macrophages, we hypothesized that Lrrk2 contributes to basal type I IFN expression upstream of cytosolic RNA or DNA sensing, two nucleic acid sensing pathways that are interconnected between positive and negative feedback loops . To directly test the involvement of cGAS in generating elevated resting levels of type I IFN in Lrrk2 KO macrophages, we crossed Lrrk2 KO and cGas KO mice and compared type I IFN transcript levels in double KO BMDMs with those of littermate controls. Although basal Isg15 expression differences between Lrrk2 KO and HET BMDMs were more modest in this experiment, loss of cGAS significantly reduced basal ISG expression in Lrrk2 KO BMDMs . With lowered resting type I IFN levels, cGas/Lrrk2 double KOs were able to respond normally to IFN/ISG-generating innate immune stimuli like DMXAA, which bypasses cGAS and stimulates STING directly , and poly transfection . Consistent with the ability of cGas ablation to rescue Lrrk2 KO baseline and induction defects, western blot analysis showed that levels of STAT1 phosphorylation were restored in cGas/Lrrk2 double KOs . Together, these results support a model where high basal levels of type I IFN and ISGs in Lrrk2 KO macrophages are due to chronic engagement of the cGAS-dependent DNA sensing pathway.
Cytosolic mtDNA drives basal type I IFN expression in Lrrk2 KO macrophages.
Study Examines Connection Between Diabetes Medication And Parkinsons Disease
It was first suggested in the 1960s that people with type-2 diabetes are at increased risk for developing Parkinsons disease and when they do develop PD, its progression is faster and often more severe. This may be due, in part, to an apparent relationship in the brain between dopamine, insulin resistance, and glucose control. Insulin is not only made in the pancreas, its also present in the brain where it has been shown to impact dopamine levels.
Parkinsons is generally believed by scientists to be caused by the loss of dopamine-producing neurons. Parkinsons symptoms, such as slowness, rigidity, and tremor, typically develop after approximately 40-80% of these dopamine-producing neurons die.
Why does this matter? Currently, more than 30 million people in the United States have type-2 diabetes, and that number is growing. The lifetime is also on the rise. In light of these trends, it would be valuable to know whether any specific type-2 diabetes medications might be associated with an increased or decreased risk for developing PD.
1) Thiazolidinediones , like pioglitazone or rosiglitazone , which specifically target insulin resistance
2) Drugs, like albiglutide or dulaglutide , that mimick glucagon-like peptide-1 a hormone that promotes insulin secretion, and
3) Dipeptidyl peptidase 4 inhibitors, which increase GLP-1 levels, and lead to insulin secretion and lowering of blood sugar levels
Results
Learn More
Read Also: What Percentage Of Parkinson’s Patients Develop Dementia
