Blood Flow And Glucose Metabolism
Blood flow and glucose metabolism dissociation in subcortical regions, especially putamen, has been found implicated in the occurrence of LID . Blood flow increased while glucose metabolism decreased in the putamen, be it in the medicated or unmedicated state . Bilateral cerebellum cTBS alleviates LID by reducing -fluorodeoxyglucose positron emission tomography metabolism in bilateral cerebellar hemispheres and dentate nucleus . This study suggests that metabolic changes might mediate the efficacy of TMS . Till now, none studies have unraveled the relation of blood flow with TMS in the management of LID. Nevertheless, many studies indeed identified blood flow alteration after TMS in a wide range of brain regions and various diseases. Blood flow and glucose metabolism may imply some beneficial effects of TMS on LID.
You Are Not Alone: Finding Support For Parkinsons And Dyskinesia
How does dyskinesia affect your daily life? Has your doctor or neurologist found the right medication regimen to reduce your symptoms? What helps you successfully get through each day?
Share your tips and experiences in a comment below or on MyParkinsonsTeam. You’ll be surprised how many other members have similar stories.
Brain Regions In Associative And Limbic Basal Ganglia Loop
Voxel based morphometry reveals increased gray matter volume of the bilateral IFC in dyskinetic patients . Right IFC engages in suppressing an already initiated manual response . One study further revealed dyskinetic PD patients have a weaker inhibitory interaction between the right IFC and contralateral MC . This finding conforms with beneficial effects of inhibitory cTBS over right IFC on LID . Another study revealed that connectivity of the right IFC with the left MC was decreased in patients with LID . Nevertheless, inhibitory cTBS over right IFC improved LID symptoms in this study as well . Authors speculated that the increased communication between the right IFC and the putamen observed in this study in patients with LID might interfere with the motor inhibition network .
Task-based functional magnetic resonance imaging revealed activation of pre-SMA after intake of levodopa in LID patients . The pre-SMA has been implicated in both the suppression and initiation of movements . This might partly explain the contradictory outcomes of two LF-rTMS studies over pre-SMA on LID .
Activation of the dorsolateral prefrontal cortex was also observed in PD patients with LID . However, it was bewildering that HF-rTMS Stimulation of the left DLPFC induced a significant MC depression . Moreover, such MC depression did not reach a significant reduction of LID symptoms .
Read Also: What Is The Life Expectancy Of Someone With Parkinson’s Disease
Paradox Of Dyskinesias And Parkinsons Disease
Dyskinesias usually appear at a turning point in the course of treating PD, when the disease is advancing. Generally, as the disease advances and symptoms worsen, there is a need to increase the dosage of levodopa. But increasing the dose of levodopa is associated with a worsening of dyskinesias. Reducing the dosage in an effort to reduce dyskinesias leads to poor control of PD. Some patients find they prefer the movement associated with dyskinesias to the immobility they experience when their PD symptoms arent well controlled.1
What Does Dyskinesia Look Like
Levodopa-induced dyskinesia causes symptoms ranging from writhing or wriggling to dramatic rocking and head bobbing, from minor tics to full-body movements. Dyskinesia can also cause swaying, which can be embarrassing on its own and even more so when youre walking. Some people with dyskinesia fear others will think theyre intoxicated and, combined with other Parkinsons symptoms such as freezing of gait, rigidity, and balance problems, walking around in public can feel too vulnerable to do.
Read Also: What Is The Life Expectancy Of Someone With Parkinson’s Disease
Analysis Of Incident Lid Risk Vs Candidate Snca Variants
After investigating non-genetic variables, we analyzed the effect on LID onset of two SNCA genetic variants increasing the expression levels of the gene, namely rs356219 and D4S3481 . To this end, we built multivariable Cox PH regression models adjusted for the non-genetic variables retained in the stepwise regression above. This analysis was driven by the hypothesis that these markers could affect LID onset, as previously suggested for other SNCA variants . Specifically, we tested these associations under the assumptions of additive effect for the SNP rs356219, as previously tested by Kim et al. , and of a dominant effect for the microsatellite marker D4S3481, where carriers of the 263 bp allele were tested against non-carriers, as in Corrado et al. . A Bonferroni correction for two independent genetic variants tested was applied, resulting in = 0.025.
To Best Understand Dyskinesia You First Have To Know About Dopamine
Dopamine is a chemical that sends signals between the nerve cells of your brain. These signals help make voluntary movement happen.
As a person with Parkinson’s disease, your brain produces less dopamine over time. This affects how you move and is why you experience tremors, slowness, and other Parkinson’s disease symptoms.
To treat Parkinson’s disease, many doctors use a medication called levodopa, which helps replenish the lack of dopamine in the brain. Increasing dopamine can help ease many of Parkinson’s symptoms. This is called GOOD ON time, also known as ON time without troublesome dyskinesia.
GOOD On time
This occurs when PD medication is working as expected. PD symptoms are well-controlled and people with Parkinson’s are not experiencing dyskinesia
Levodopa works well in treating Parkinsonâs diseaseâoften for several years. As Parkinsonâs disease progresses, levodopa can wear off sooner between doses. At these times, symptoms worsen. This is called OFF time.
OFF time
When your medication, like levodopa, wears off throughout the day and tremors, slowness, and other Parkinson’s disease symptoms return. They may include shakiness and jitters, slowed movement, and difficulty trying to stand
When first initiated on levodopa therapy, Parkinson’s disease symptoms are well-controlled, and these benefits can last for several hours. This is when you experience GOOD ON time.
You May Like: Md Symptoms In Adults
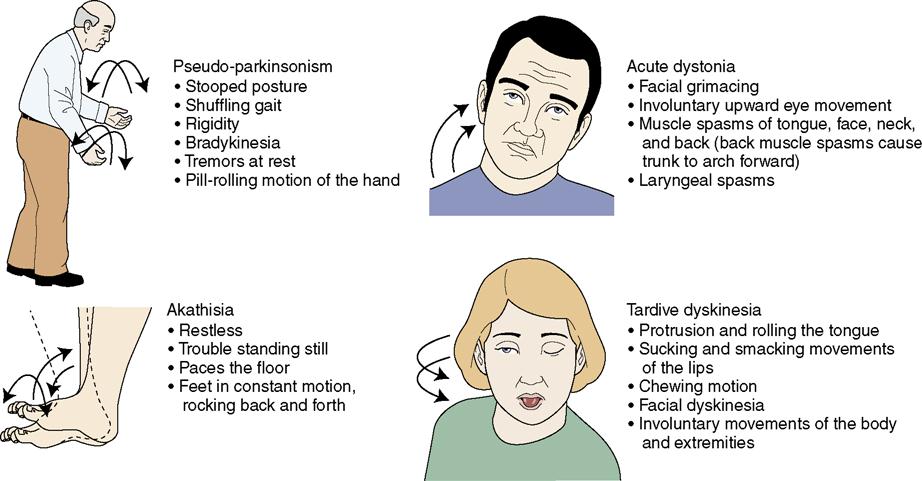
What Does Tardive Dyskinesia Look Like
TD looks like different, uncontrollable movements and patterns of the limbs and face. Sometimes referred to as stereotypy, the activity can be patterned, repetitive, and rhythmic movements that can involve one or more body parts. More than 3/4 of those with TD experience oral-facial-lingual stereotypic movements .1 The Baylor College of Medicine Movement Disorders Clinic conducted a videotape review of 100 people with tardive dyskinesia. The evaluation showed that the majority experienced irregular and chaotic movements in the OFL region, including lip smacking, chewing and other tongue and mouth movements. Other areas of the body can also show signs of TD like nodding and rocking, repeated body movements like crossing and uncrossing arms and legs, and random vocalizations.
Those who experience these involuntary movements may not even realize it. Like other conditions, these stereotypies can get worse under stress. They can manifest as muscle contractions or spasms, inability to be still, facial tics, or other jerking and abnormal movements.
Drugs Acting On Serotonergic Systems
The basal ganglia have dense serotonergic innervation. It is suggested that serotonergic transmission has an inhibitory effect on dopaminergic transmission. There are reports of successful use of 5HT agents in treating LIDs., However, these studies included very small numbers and were mostly uncontrolled.
Don’t Miss: What Causes Dyskinesia In Parkinson’s
Acknowledgements And Conflict Of Interest Disclosure
RM acknowledges grants from the Spanish Ministries de Economía y Competitividad and of Sanidad Política Social e Igualdad, ISCIII: BFU2010-20664, PNSD, CIBERNED ref. CB06/05/0055 and Comunidad de Madrid ref. S2011/BMD-2336, JRGM is supported by ICyTDF México MTH acknowledges the support by CIBERNED CB05/05/505, SAF2007-062262 and FIS PI10-02827. RH and KC were supported by the German Bundesministerium für Bildung und Forschung, Grant 01GN1006B. NS gratefully acknowledges Sardinia Regional Government for financial support . The authors have no conflicts of interest to declare.
All experiments were conducted in compliance with the ARRIVE guidelines.
Box 1classification Of Levodopainduced Dyskinesias
-
Peak dose dyskinesia
-
On state dystonia
-
Yoyoing
-
Peakdose dyskinesiasThese are the most common forms of LID and are related to peak plasma levels of levodopa. They involve the head, trunk, and limbs, and sometimes respiratory muscles. Dose reduction can ameliorate them, frequently at the cost of deterioration of parkinsonism. Peakdose dyskinesias are usually choreiform, though in the later stages dystonia can superimpose.
-
Diphasic dyskinesiasThese develop when plasma levodopa levels are rising or falling, but not with the peak levels. They are also called DID . DID are commonly dystonic in nature, though chorea or mixed pattern may occur. They do not respond to levodopa dose reduction and may rather improve with high dose of levodopa.
-
Off state dystoniasThese occur when plasma levodopa levels are low . They are usually pure dystonia occurring as painful spasms in one foot. They respond to levodopa therapy. Rare forms of LID include on state dystonias and yoyo dyskinesia .
Read Also: What Essential Oils Are Good For Parkinson’s Disease
Parkinson’s Dyskinesia Mechanism Explained
- Scripps Research Institute
- Summary:
- The mechanism underlying Parkinson’s dyskinesia has been unknown, until now. An international collaboration has found a key cause, and with it, potentially, a new route to providing relief.
Many people with Parkinson’s disease eventually develop debilitating movements called dyskinesia, a side effect of their much-needed dopamine replacement medication. The mechanism underlying this unwanted side effect has been unknown, until now. An international collaboration led by Scripps Research, Florida has found a key cause, and with it, potentially, a new route to providing relief.
Dopamine replacement therapy makes Parkinson’s symptoms much better at first, but eventually treatment gives way to uncontrollable, jerky body movements. But why? New research shows that underlying this development is the therapy’s unintended boost of a protein with the unwieldy name Ras-guanine nucleotide-releasing factor 1, or RasGRP1 for short. This boost in RasGRP1 produces a cascade of effects which lead to abnormal, involuntary movements known as LID, or L-DOPA-induced dyskinesia, says co-lead author Srinivasa Subramaniam, PhD, associate professor of neuroscience at Scripps Research, Florida.
Encouragingly, the collaboration found that in dopamine-depleted mice and other animal models, inhibiting production of RasGRP1 in the brain during dopamine replacement diminished the involuntary movements without negating the useful effects of the dopamine therapy.
Clinical Features And Classification Of Lid
LID are clinically heterogeneous. They commonly present as chorea or choreoathetosis, though myoclonus, akathasia, ballism and other forms of abnormal movements have also been described. LID generally first appear on the side worst affected by Parkinson’s disease and in legs before arms. This could be related to an early dopaminergic loss in the dorsolateral striatum, the region corresponding somatotopically to the foot area.
Chorea refers to involuntary, rapid, irregular, purposeless, and unsustained movements that seem to flow from one body part to another. The severity of these movements can vary from occasional abnormal movements that are absent at rest and provoked only during active movementfor example, walking or talking to violent large amplitude flinging and flailing arm movementsthe ballism. Often, there are superimposed writhing athetoid movementschoreoathetosis. Dyskinesias may predominantly affect particular body partsfor example, torso, head and neck, limbsor speech or respiratory muscles.
Dystonia is the second most common form of LID presenting as sustained muscle contractions. It occurs either in pure form or in combination with the chorea, in the latter case manifesting as twisting of the leg on walking or the arm being pulled behind the back. Dystonia accounts for greater disability than chorea. Off time dystonias are usually painful.
You May Like: Parkinson’s Mortality Rate
Take Additional Medication For Your Parkinson’s Disease
Taking a medication called a dopamine agonist can allow your doctor to reduce your levodopa dosage, which may help ease the symptoms of dyskinesia. However, according to the 2016 review, these drugs can cause similar side effects as those of levodopa for some people and may require you to adjust your dose of levodopa.
Adding amantadine to your treatment regimen may also provide relief of dyskinesia symptoms.
Cannabinoids And Their Role In Dyskinesia
In addition to dopamine, other neurotransmitter systems modify dopamine receptor-signaling cascades involved in dykinesia. Growing evidence implicates the endocanabinoid system in dyskinesia, since CB1R are highly expressed in both D1R-and D2R-containing MSN, and negatively modulate D1R- and D2R-mediated behaviors . This is the main reason to postulate CB1R as a therapeutic target to control the imbalance of GABAergic or glutamatergic neurons in PD and dyskinesia. There is also evidence that endocannabinoids and cannabinoid agonists inhibit dopamine reuptake by blocking dopamine transporters and thus may have implications for the fine tuning of the striatal circuitries involved in dyskinesia .
Cannabinoid agonists have shown antidyskinetic effects in animal models of PD and antagonists may have antidyskinetic properties as well . However, only cannabinoid agonists decrease dyskinesia in clinical studies . These paradoxical effects may be explained in part by the modulation of CB1R and vanilloid TRPV1R by endogenous cannabinoids such as oleoylethanolamide , which binds the peroxisome proliferator-activated R and TRPV1R.
You May Like: Does Sean Penn Have Parkinson’s Disease
Brain Regions In Motor Basal Ganglia Loop
MC is a crucial brain region involving in the development of LID. Alterations of potentials recorded from MC shed light on possible mechanisms underlying the benefits of LF-rTMS and cTBS for LID.
Short-interval intracortical inhibitions and long-latency intracortical inhibition reflect suppression of MC excitability . In off therapy, SICI and LICI were decreased in PD patients with and without LID . Unlike PD patients without LID, administration of levodopa could not reverse decreased SICI and LICI in PD patients with LID . In off therapy, -Aminobutyric acid agonist increased SICI in PD patients . Administration of GABAergic agonist could also alleviate LID . It is believed that SICI is likely to be mediated by GABA-A-ergic receptors and LICI by GABA-B-ergic receptors .
On the contrary, intracortical facilitation and short-interval intracortical facilitation reflect the facilitation of MC excitability . Regardless of drug condition, ICF was found to decrease or remain normal in PD patients with LID . Unlike ICF in dyskinetic patients, SICF kept increased in off and on the state . Such increase was positively correlated with the severity of LID . Increased SICF in LID patients could be alleviated by anti-glutamatergic drugs . Improvement of LID did not come with restoration of SICF , which suggests additional pathophysiological mechanisms might contribute to LID.
Risk Factors For Developing Dyskinesia
A study published in 2018 assessed the risk factors of levodopa-related dyskinesia in a group of Parkinsons disease patients enrolled in the Parkinsons Progression Markers Initiative . The study identified seven independent risk factors associated with the development of levodopa-related dyskinesia. These factors were: female sex, greater exposure to levodopa treatment, severe motor and functional impairment, lack of tremors, a higher genetic risk score which is based on the analysis of the presence or absence of variations in multiple genetic locations that are associated with the disease, anxiety and marked asymmetric or non-uniform distribution of a domapine transporter protein in an area of the brain called the caudate region.
Don’t Miss: What Are Early Warning Signs Of Parkinson’s Disease
Change Your Levodopa Dose
Parkinson’s symptoms happen when you donât have enough dopamine, a brain chemical that helps your limbs move smoothly. Levodopa is a drug that raises the amount of dopamine in your brain. It prevents stiffness and jerky movements.
When you take levodopa, the amount of dopamine in your brain goes up. As the drug wears off, those levels drop. These up-and-down changes may be part of what causes dyskinesia.
One way to prevent the condition is to lower the dose of levodopa you take. The challenge is to lower it just enough to avoid this side effect but still take enough of the drug to control your Parkinson’s symptoms. Your doctor can help you fine-tune your dose. They may also add other types of medications to your treatment.
Another option is to switch to an extended-release form of levodopa. The medicine releases more slowly into your blood to keep your dopamine level steadier.
Are There Ways To Manage Dyskinesia
Once dyskinesia has started it is difficult to treat. However, there are several ways to delay it from starting or reduce it once it has begun.
Supplemental or alternative treatment options
Things you can do on your own
- Keep a diary that logs the time and frequency of dyskinesia, which will help your doctor assess if your medications are working and help you schedule daily activities when mobility is better.
- Physical activity, including mild aerobic exercise such as walking, dancing, and swimming, will help keep the body strong and prevent muscle weakening.
- Stress can make dyskinesia symptoms worse, so find ways to reduce stress and try to keep a positive attitude.
- Poor sleep at night is associated with dyskinesia. Aim for good sleep quality and try to experiment with different positions in bed that will help you relax and sleep better.
Don’t Miss: When Was Parkinson’s Disease First Discovered
What Makes Dyskinesia Worse For Members
People with Parkinsons disease lose brain cells that produce the chemical dopamine. Levodopa therapy relieves Parkinsons symptoms by temporarily replacing dopamine, but fluctuating levels of levodopa cause dyskinesia as a side effect. This fluctuation is believed to contribute to the motor complications that characterize dyskinesia.
Dyskinesia can occur or worsen at two different points. One is when dopamine levels are at their highest, called peak-dose dyskinesia, usually one or two hours after medication is taken. The other is when dopamine levels drop, called diphasic dyskinesia.
I start to feel dyskinesia about a half-hour to an hour after my morning dose, said one member, whose symptoms subside after two hours. Mine hits whenever the meds are wearing off and can last up to two hours a stretch, explained another. My symptoms are worse in the afternoon and evening , said one member.
Some members of MyParkinsonsTeam said dyskinesia lessened or stopped when their neurologists reduced their levodopa dosage or prescribed a drug approved by the U.S. Food and Drug Administration to treat it such as Gocovri, an extended-release formulation of the drug amantadine.
When my doctor lowered the dose of Sinemet , the dyskinesia disappeared, said one member. We reduced my wifes dose and the dyskinesia virtually disappeared, agreed another.