What Determines Who Gets Parkinson’s Disease
In most cases inheriting a non-working copy of a single gene will not cause someone to develop Parkinson’s disease. We believe that many other complicating factors such as additional genes and environmental factors determine who will get the condition, when they get it and how it affects them. In the families we have studied, some people who inherit the gene develop the condition and others live their entire lives without showing any symptoms. There is a lot of research on genes and the environment that is attempting to understand how all these factors interact.
Genetic Testing in Parkinson’s Disease
Genetic testing has recently become available for the parkin and PINK1 genes. Parkin is a large gene and testing is difficult. At the current stage of understanding, testing is likely to give a meaningful result only for people who develop the condition before the age of 30 years. PINK1 appears to be a rare cause of inherited Parkinson’s disease. A small percentage of those developing the condition at an early age appear to carry mutations in the PINK1 gene. Genetic testing for the PARK7, SNCA and LRRK2 genes is also available.
Additional Resources
Genetic Risk For Parkinson’s Disease
If you have a genetic mutation associated with Parkinson’s, will you get the disease? Not necessarily. Some mutations carry a greater risk, but;none bring a 100 percent chance of developing Parkinson’s disease. There are many Parkinson’s risk genes where a mutation means a very small increased likelihood of Parkinson’s.;Researchers are looking for other factors; that either push or protect someone with a gene mutation to or from having Parkinson’s. Your doctor and/or a genetic counselor can discuss the risk associated with different Parkinson’s genes and what your results may mean for you and your loved ones.
What Raises Someone’s Risk For Parkinson’s
It’s a complex picture, but you may be more likely to get Parkinson’s based on:
Age. Since it mostly affects people 60 and older, your risk goes up as the years go by.
Family history. If your parent, brother, or sister has it, you’re a little more likely to get it.
Job. Some types of work, like farming or factory jobs, can cause you to have contact with chemicals linked to Parkinson’s.
Race. It shows up more often in white people than other groups.
Serious head injury. If you hit your head hard enough to lose consciousness or forget things as a result of it, you may be more likely to get Parkinson’s later in life.
Gender. Men get it more than women. Doctors aren’t sure why.
Where you live. People in rural areas seem to get it more often, which may be tied to chemicals used in farming.
Don’t Miss: What Color Is The Ribbon For Parkinson’s
New Genes For Recessive And X
Compared to monogenic dominant PD and to the well-established recessive early-onset PD genes PARK2, DJ-1, and PINK1, the newly identified recessive forms appear more complex both for clinicians and researchers. The clinical picture of the newly identified recessive forms is often more severe and multifaceted. However, in a few instances, there appears to be a genotype-phenotype correlation where mutations that lead to pronounced alteration of normal protein function cause a complex disorder with severe additional neurological or neuropsychiatric impairment, often from birth, and juvenile Parkinsonism, whereas mutations with a milder effect on the protein cause Parkinsonism with fewer atypical features. Table 2 summarizes currently known and putative genes for recessive and X-linked PD or Parkinsonism.
Table 2 Monogenic causes for autosomal recessive or X-linked Parkinsons disease or atypical juvenile Parkinsonism
Genetics: Insights Into Etiology
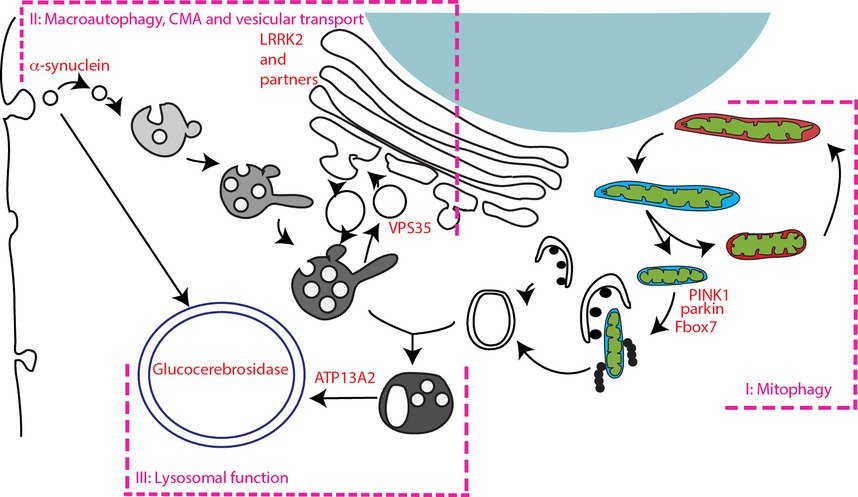
Improvement in genetic analysis techniques in the 1990s led to the discovery of the first genetic cause of PD: mutations in the SNCA gene encoding -synuclein . At around the same time, -synuclein was found to be the major constituent of LB, the pathological hallmark of PD . Subsequently, multiplications of the SNCA gene have been found to cause PD with penetrance increasing with gene dosage . These discoveries brought -synuclein to center stage in the study of the pathogenesis of PD and led to the hypothesis that during different stages of the disease, -synuclein spreads in a stereotypical way within the nervous system in a prion-like fashion .
Table 2 Examples of genes associated with PD risk
Read Also: What Color Is The Ribbon For Parkinson’s
Can Parkinsons Disease Be Prevented
Unfortunately, no. Parkinsons disease is long-term disease that worsens over time. Although there is no way to prevent or cure the disease , medications may significantly relieve your symptoms. In some patients especially those with later-stage disease, surgery to improve symptoms may be an option.
People Who Already Have Pd: Should I Get Tested And What Do I Do With The Results
Up until recently, even people with PD with a very extensive family history of PD would not necessarily receive genetic testing because there were no clear uses for the results. There has been research directed at figuring out whether PD caused by or associated with certain mutations have particular clinical characteristics . However, there remains so much variability in clinical characteristics even among people with the same PD mutation, that there are still no clear practical implications in knowing whether a PD patient harbors a particular mutation.; There is also, so far, no difference in treatment or management of PD whether or not the patient harbors one of the known mutations. That may change however, with the advent of clinical trials that target particular mutations.
There are two genes that have received particular attention recently because medications are being developed that target those with mutations of these genes.
GBAis a gene that increases the risk of developing PD. The gene encodes for the GBA enzyme, a protein used by the body to break down cellular products. Having two abnormal GBA genes causes Gauchers disease, which is characterized by the buildup of these cellular products resulting in fatigue, bone pain, easy bleeding and an enlarged spleen and liver. When a person inherits only one abnormal gene, he or she does not develop Gauchers disease, but does incur a small increased risk of PD. Most people with one mutated GBA gene do not develop PD.
Recommended Reading: Is Parkinson’s Disease Deadly
Concluding Remarks And Future
Genes implicated in Mendelian forms of PD have provided new insights into the pathogenesis of the disease. The molecular pathways identified in monogenic cases may also be implicated in sporadic PD. The effect of dosage of SNCA on the phenotype of patients with duplications or triplications is illustrative. In addition, non-coding variants in this gene, thought to affect the level of expression in neurons, are associated with risk of the disease. The molecular mechanisms that contribute to PD and related disorders result in the death of dopaminergic neurons in vulnerable brain regions, and consequently the shared phenotype. However, known PD-causing genes account for only a small fraction of monogenic forms. Robust high-density SNP genotyping technologies and data analysis programs, combined with the analysis of copy number variations and large pathogenic genomic rearrangements, will identify novel loci. The clinical heterogeneity of parkinsonism is probably the cumulative effect of different gene-environment and/or genegene interactions. To identify risk variants in PD, association study methodology must be improved. Studies in isolated and heterogeneous populations, and approaches that minimize population stratification, are needed. Large-scale studies and publicly available GWA databases, crucial for statistical power, require collaborative efforts with shared sets of stringent clinical, genetic and analytic methods.
What Genes Are Linked To Parkinson’s Disease
In 1997, we studied a large family that came from a small town in Southern Italy in which PD was inherited from parent to child . We found the gene that caused their inherited Parkinson’s Disease and it coded for a protein called alpha-synuclein. If one studies the brains of people with PD after they die, one can see tiny little accumulations of protein called Lewy Bodies . Research has shown that there is a large amount of alpha-synuclein protein in the Lewy Bodies of people who have non-inherited PD as well as in the brains of people who have inherited PD. This immediately told us that alpha-synuclein played an important role in all forms of PD and we are still doing a lot of research to better understand this role.
Currently, seven genes that cause some form of Parkinson’s disease have been identified. Mutations in three known genes called SNCA , UCHL1 , and LRRK2 and another mapped gene have been reported in families with dominant inheritance. Mutations in three known genes, PARK2, PARK7 , and PINK1 have been found in affected individuals who had siblings with the condition but whose parents did not have Parkinson’s disease . There is some research to suggest that these genes are also involved in early-onset Parkinson’s disease or in dominantly inherited Parkinson’s disease but it is too early yet to be certain.
Don’t Miss: What Essential Oils Are Good For Parkinson’s Disease
Are Genes Responsible For Monogenic Disorders Also Susceptibility Factors
Associations detected by screening candidate genes in controls and patients cannot always be replicated in follow-up studies, and few candidate genes were confirmed in meta-analysis, because of potential biases and confounding factors, including population stratification, small sample size, misclassification and/or inappropriate statistical methods. Polymorphic variants in SNCA and LRRK2 genes, and heterozygous mutations in the GBA gene, however, have been validated as genetic susceptibility factors .
Nucleotide polymorphisms located close to the promoter region and throughout SNCA have been associated with sporadic PD, although much of the data is equivocal . Rep1 , a mixed nucleotide repeat, 10 kb upstream of the translational start of SNCA , has been confirmed as a risk factor , and synergy between an SNCA variant and a polymorphism in microtubule-associated protein tau , each of which increases the risk for the development of PD, has been detected . The combination of risk genotypes in SNCA and MAPT doubles the risk of PD, further supporting the notion that the related pathways contribute to neurodegenerative diseases . The risk associated with Rep1 does not interact, however, with herbicide exposure, an independent risk factor in PD .
How Is Parkinsons Disease Diagnosed
Diagnosing Parkinsons disease is sometimes difficult, since early symptoms can mimic other disorders and there are no specific blood or other laboratory tests to diagnose the disease. Imaging tests, such as CT or MRI scans, may be used to rule out other disorders that cause similar symptoms.
To diagnose Parkinsons disease, you will be asked about your medical history and family history of neurologic disorders as well as your current symptoms, medications and possible exposure to toxins. Your doctor will look for signs of tremor and muscle rigidity, watch you walk, check your posture and coordination and look for slowness of movement.
If you think you may have Parkinsons disease, you should probably see a neurologist, preferably a movement disorders-trained neurologist. The treatment decisions made early in the illness can affect the long-term success of the treatment.
Also Check: Parkinson Disease Genes
What Are The Different Stages Of Parkinsons Disease
Each person with Parkinsons disease experiences symptoms in in their own unique way. Not everyone experiences all symptoms of Parkinsons disease. You may not experience symptoms in the same order as others. Some people may have mild symptoms; others may have intense symptoms. How quickly symptoms worsen also varies from individual to individual and is difficult to impossible to predict at the outset.
In general, the disease progresses from early stage to mid-stage to mid-late-stage to advanced stage. This is what typically occurs during each of these stages:
Early stage
Early symptoms of Parkinsons disease are usually mild and typically occur slowly and do not interfere with daily activities. Sometimes early symptoms are not easy to detect or you may think early symptoms are simply normal signs of aging. You may have fatigue or a general sense of uneasiness. You may feel a slight tremor or have difficulty standing.
Often, a family member or friend notices some of the subtle signs before you do. They may notice things like body stiffness or lack of normal movement slow or small handwriting, lack of expression in your face, or difficulty getting out of a chair.
Mid stage
Mid-late stage
Standing and walking are becoming more difficult and may require assistance with a walker. You may need full time help to continue to live at home.
Advanced stage
Genetic Testing For Parkinsons Disease
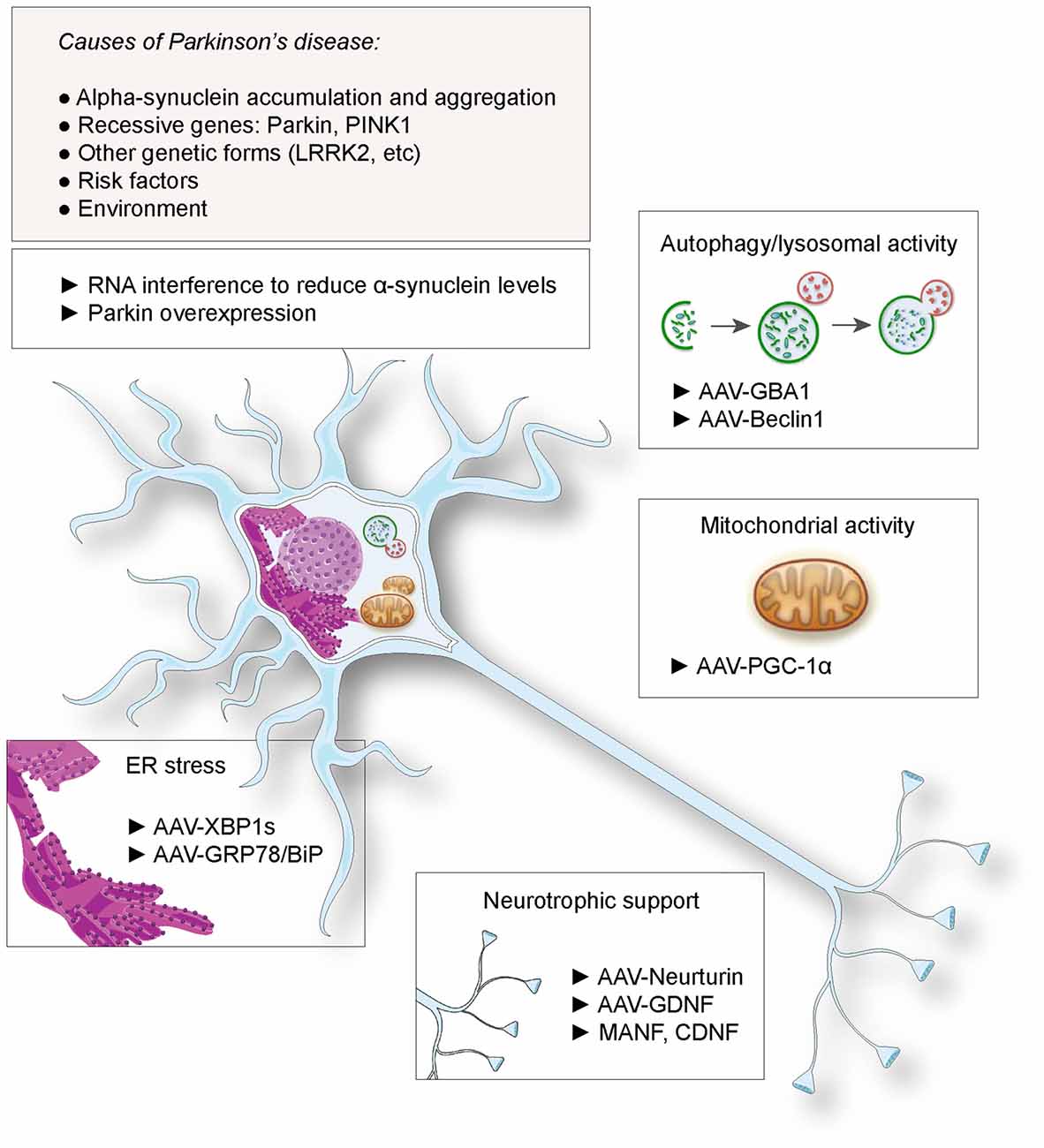
Similar to other complex diseases, the reason a particular person develops Parkinsons disease is likely a combination of genetic makeup and environment. In most people, the genetic contribution to disease development may be due to a number of different genes and the interactions between them. For only a very small percentage of people with PD, about 10%, the disease can be attributed to a single abnormal gene. Figuring out the identity and contributions of all the different genes that play a role in disease development is a very hot topic in PD research today.
Read Also: What Is The Life Expectancy Of Someone With Parkinson’s Disease
Environmental Factors: Etiological And Disease
From 1817 when Dr. James Parkinson first described 6 patients with the condition that would later bear his name , much progress was made in the following 150years. It became known that the SN was the site affected by PD pathology together with the presence of cytoplasmic inclusions, and levodopa became available as the first effective symptomatic drug treatment of PD in 1960s . However, the etiology of PD remained elusive.
Table 1 Examples of environmental factors and their biologic correlates
The most robust beneficial environmental factor associated with PD is cigarette smoking which has been seen in early case-control studies and then confirmed in more recent large prospective cohorts. Active smokers have 50% lower risk of PD compared to never smokers . There is a strong dose-response relationship including duration, intensity, pack-years and years since last smoking: PD risk decreases with increasing duration of smoking and increases again with time since quitting . Preliminary reports also support an inverse relationship between passive smoking, smokeless tobacco use and PD .
Causes Of Parkinson’s Disease
| This article may be too technical for most readers to understand. Please help improve it to make it understandable to non-experts, without removing the technical details. |
Parkinson’s disease is a degenerative disorder of the central nervous system. Most people with PD have idiopathic Parkinson’s disease . A small proportion of cases, however, can be attributed to known genetic factors. Other factors such as environmental toxins, herbicides, pesticides, and fungicides, have been associated with the risk of developing PD, but no causal relationships have been proven.
Also Check: Cardinal Signs Of Parkinson’s Disease
What Is The Outlook For Persons With Parkinsons Disease
Although there is no cure or absolute evidence of ways to prevent Parkinsons disease, scientists are working hard to learn more about the disease and find innovative ways to better manage it, prevent it from progressing and ultimately curing it.
Currently, you and your healthcare teams efforts are focused on medical management of your symptoms along with general health and lifestyle improvement recommendations . By identifying individual symptoms and adjusting the course of action based on changes in symptoms, most people with Parkinsons disease can live fulfilling lives.
The future is hopeful. Some of the research underway includes:
- Using stem cells to produce new neurons, which would produce dopamine.
- Producing a dopamine-producing enzyme that is delivered to a gene in the brain that controls movement.
- Using a naturally occurring human protein glial cell-line derived neurotrophic factor, GDNF to protect dopamine-releasing nerve cells.
Many other investigations are underway too. Much has been learned, much progress has been made and additional discoveries are likely to come.
Identification Of New Genes And Risk Factors For Pd
New PD-linked genes or PD risk factors can be identified by gene mapping or candidate gene approaches. Gene mapping in human diseases is the localization of genes underlying the clinical phenotypes of the disease on the basis of correlation with DNA variants , without the need for prior hypotheses about biological function. Genetic mapping methods include linkage analysis and genome-wide association studies. Alternatively, based on their known function, levels of expression, or mode of interaction , some genes can be considered plausible candidates, and as such, tested for in cohorts of patients.
You May Like: How To Use Hemp Oil For Parkinson’s
How Do Lrrk2 Gene Mutations Cause Parkinsons Disease
Healthy Individuals withoutLRRK2 Parkinsonâs disease
All humans have two copies of theLRRK2 gene which produce LRRK2 protein. In healthy people both copies of the gene are normal and therefore produce normal LRRK2 which regulates normal cellular biology.
Patients withLRRK2 Parkinsonâs disease
Patients withLRRK2 Parkinsonâs disease usually have one normal gene and one mutated gene. This means that patients produce both healthy LRRK2 protein and an overactive form of LRRK2 protein. The overactive LRRK2 is believed to drive neurodegeneration, causing Parkinsonâs disease, while the healthy LRRK2 continues to provide normal regulation to cells in other organs such as the lungs and kidneys.
Patients withLRRK2 Parkinsonâs disease â Non specific inhibition
In order to slow or stop the disease, ESCAPE Bio believes that overactive LRRK2 needs to be nearly fully inhibited. However, if healthy LRRK2 is also inhibited by the same amount then it may not be able to regulate cellular function in other organs, thereby driving histopathological changes in the lung and kidney, which are hard to detect and may be associated with irreversible pathology if they go unchecked.
Patients withLRRK2 Parkinsonâs disease â ESCAPEâs approach
Some Are Calling Parkinsons A Man
Researchers are rapidly coming to the viewpoint that a large number of Parkinsons cases are tied to toxins. These researchers are even reaching conclusions that environment outranks genetics as a cause of Parkinsons.
One 2020 book discussed an exhaustive study of 17,000 twin brothers to pinpoint the effects that environment could play. The researchers found that people exposed to certain environmental factors were more than twice as likely to develop Parkinsons.
Don’t Miss: End-stage Parkinson Disease What To Expect
