Er Stress And Pd Pathogenesis
Activation of the ER stress response was also reported in human PD brain. Accumulation of ER chaperons was found in LBs while increased PERK/p-eIF2 signaling was demonstrated in dopaminergic neurons of the substantia nigra in post-mortem tissue from PD cases, confirming that PD pathology is intimately associated with activation of ER stress in vivo . Interestingly, at least two protective mechanisms against ER stress have been shown to involve modulation of genes such as Parkin and LRRK2, whose mutated forms have been associated with familiar cases of PD. Parkin, an E3 ubiquitin ligase implicated in the regulation of mitophagy, was found increased after treatment with ER or mitochondria stressors and this increase was mediated directly by ATF4 binding to the parkin promoter . Overexpression of parkin protected cells from ER stress by promoting splicing of XBP-1 and the induction of the UPR prosurvival response . Also mutations causing loss of function of LRRK2, a protein involved in maintaining neuronal cellular stability, have shown to abrogate upregulation of BiP/grp78 level after 6OHDA treatment or overexpression of S, enhancing neuronal death in vitro and in vivo . Thus, additional protective mechanisms may be important in preserving cellular environment from detrimental effects of ER stress whereas alteration in such pathways may contribute to PD progression.
What Is The Clinical Status Of Cell
The first in-human clinical trials using iPS cell-derived dopamine-producing cells were approved to begin in Japan in August 2018, and trials in the U.S. using embryonic stem cell-derived neurons may start in late 2018 or early 2019. In Europe, similar work is likely to enter human trials for the first time around 2021. Trials in Australia using a non-embryonic stem cell source began in 2016, but issues relating to aspects of this trial have been raised, including the origin of the cells, the type of cells transplanted, and the availability and transparency of pre-clinical data.
A number of research groups are working together to understand and find potential cures for PD, and their global alliance is at the forefront of several of the experimental stem cell-based trials currently underway, including those in Japan, the U.S., and Europe. Such international collaborations can be important in accelerating research and coordinating tests for safety and efficacy of new potential cell-based PD therapies.
Concluding Remarks: Sorting Facts From Artifacts In Pd
Second, further effort is needed to integrate the various proximal pathophysiological mechanisms that have been implicated in PD. For instance, does -Syn aggregation contribute to oxidative stress and vice versa? Do these stresses reinforce each other in a loop? What about the relationships between these and all the other potential proximal events discussed here? Due to their inter-connectedness, it may be that irrespective of the initiating causes of neurodegeneration in PD, the same sets of proximal cellular responses will be ultimately activated.
Third, more work is needed to clarify the disease-relevant links between the more proximal insults and distal cell death pathways. Most of the work in this area has utilized toxin-based models. However, numerous in vitro and in vivo systems are now available that model PD based on more relevant gene abnormalities. Testing the importance of the distal pathways discussed in this review, such as JNK, p53, and specific Bcl2 family members, in these gene-based models would add important information regarding the relevance of these pathways to PD.
How Is Parkinsons Disease Currently Treated
Since many of the major features of PD are caused by the loss of neurons that release dopamine, treatment focuses on replacing dopamine or imitating its effects with drugs that mimic its actions that have produced great clinical benefit since the 1960s. However, over time these drugs create significant side effects of their own, including erratic motor responses and involuntary movements as well as neuropsychiatric problems. Thus, these drugs, which need to be consistently taken, create problems that ultimately may be as problematic as the disease they are being used to treat.
For later, more advanced stages of the disease, invasive deep brain surgical stimulation may also be used, but is not without risks, and, like the pharmaceutical options, may offer only temporary symptomatic relief.
Er Protein Folding And Calcium Storage Functions Are Prominent Sources Of Ros
The endoplasmic reticulum is the site of secretory protein production and post-translational modifications such as protein folding and glycosylation. Protein folding is a process that is greatly affected by the redox status of the ER lumen as the formation of disulfide bonds requires a highly oxidizing environment. During disulfide bond formation, electrons are transferred from the target protein to oxygen by protein disulfide isomerase and ER oxidoreductin-1, which forms ROS as byproduct . Quantitative analyses of protein synthesis and processing suggest that disulfide bond formation produces approximately 25% of total ROS in the ER lumen .
Environmental Factors And Exposures
Exposure to pesticides and a history of head injury have each been linked with PD, but the risks are modest. Never having smoked cigarettes, and never drinking caffeinated beverages, are also associated with small increases in risk of developing PD.
Low concentrations of urate in the blood is associated with an increased risk of PD.
Drug-induced parkinsonism
Different medical drugs have been implicated in cases of parkinsonism. Drug-induced parkinsonism is normally reversible by stopping the offending agent. Drugs include:
Aspirin Targets A Pathway To Cell Death In Parkinsons Disease
A new laboratory study finds that salicylic acid, the substance that gives aspirin its medicinal effects, blocks a molecular process that leads to brain cell death in Parkinsons disease and other neurodegenerative diseases. The results appear in the November 25 edition of PLOS ONE.
Like many medicines, the active ingredient in aspirin was originally derived from plants. Daniel F. Klessig, Ph.D., a plant biologist at Cornell University was studying this substance and its role in plants when he noticed that it binds to an enzyme called GAPDH , which when activated can cause cell death. He knew that humans have the enzyme too, and that a PD drug called selegiline is also able to bind to it.
He and his colleagues wondered whether salicylic acid might also bind to GAPDH in humans, and whether it might have similar effects on PD as Deprenyl.
They used a laboratory technique called high-throughput screening, which can quickly assess whether a substance interacts with hundreds of other chemicals. First they used the technique to see whether salicylic acid binded to any proteins in human cells. They not only tested regular salicylic acid, they also looked at synthetic forms and forms derived from the Chinese medicinal herb licorice.
Lastly, they investigated whether any of the substances regular salicylic acid, synthetic forms or forms derived from licorice could prevent cell death by testing them in human cells .
Results
What Does It Mean?
Reference
Potential Risk Factors In Pd
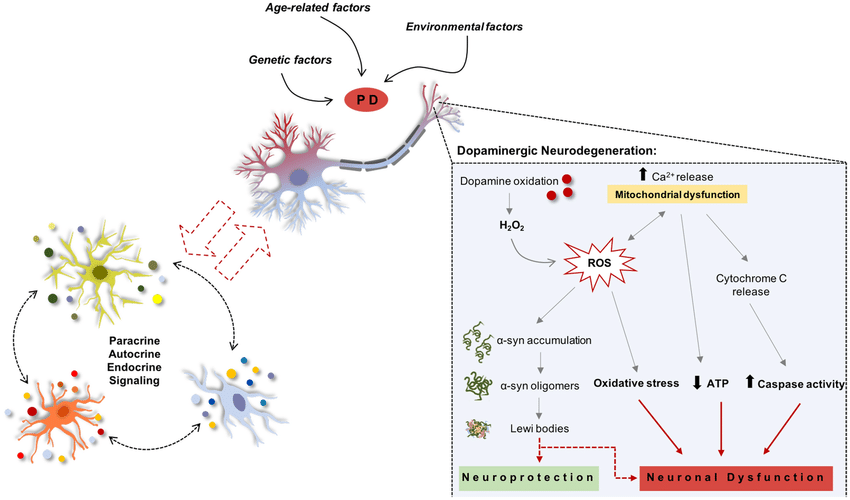
Age is one of the prominent risk factors in PD . Studies have shown that dopaminergic neuronal populations appear selectively susceptible to loss with ageing compared to many other brain regions and those related to other neurodegenerative disorders . Furthermore, studies have also shown that the dopaminergic neurons are particularly vulnerable to the mitochondrial dysfunction with advancing age .
Principle Behind Histone Modification And The Different Types Of Histone Modifications
Histones are proteins that pack and order DNA into nucleosomes. Each nucleosome contains two subunits each of histones H2A, H2B, H3, and H4, known as the core histones . A 147-bp segment of DNA wrapped around the histone octamer and neighbouring nucleosomes are separated by, on average, 50 bp of free DNA. Histone H1 is termed the linker histone, and it does not form the integral part of the nucleosome. However, it binds to the linker DNA , sealing off the nucleosome at the location where DNA enters and leaves.
7.1.1. Histone acetylation/deacetylation
Histone modifications such as acetylation and deacetylation play important roles in gene regulation. These are associated with transcriptional activation and repression respectively . Histone acetylation is a reversible process. Acetylation is catalysed by histone acetyltransferases , which are categorized into three families . HATs catalyse acetylation via the transfer of an acetyl group from acetyl-coenzyme A to the -amino group of lysine side chains on the N-terminal tails of H2A, H2B, H3, and H4 . It has recently been shown that HATs can catalyse acetylation at lysine 56 within the core domain of H3 . Histone deacetylation is performed by a class of enzymes known as histone deacetylases . These HDACs remove the acetyl groups from the -amino group of lysines. HDACs are classified into four classes based upon sequence homology and cofactor dependencies.
7.1.2. Histone methylation/demethylation
Ermitochondria Signaling In Neurodegeneration
Neurodegenerative diseases including PD, AD, and ALS/FTD share several obvious features: they are characterized by progressive nervous system dysfunction, affect millions of people worldwide and there is still no cure for any of them. Furthermore, despite affecting different brain regions PD, AD, and ALS/FTD also share other characteristics suggesting that common cellular processes may converge.
Thus, whilst the precise mechanisms remain to be determined, a variety of cellular processes are damaged in all of them, including Ca2+ dysregulation, defects in axonal transport, neuroinflammation, loss of cellular proteostasis and mitochondrial dysfunction,,,,, . Remarkably, ERmitochondria associations, regulates all of those processes. The findings that alterations in ERmitochondria associations occur in neurodegenerative diseases have given rise to the hypothesis that damaged ERmitochondria signaling is a common potential therapeutic target amongst distinct age-dependent neurodegenerative disorders.
Fig. 3: Proposed model for endoplasmic reticulummitochondria signaling in PD
ERmitochondrial axis appears to be essential for the healthy neurons. Conversely, the disruption of this interaction may involve the develop of some processes as: mitochondrial dysfunction, induction of oxidative stress, calcium dyshomeostasis, autophagy defects or neuroinflammation, which induce neuronal damage and trigger neurodegenerative diseases as PD
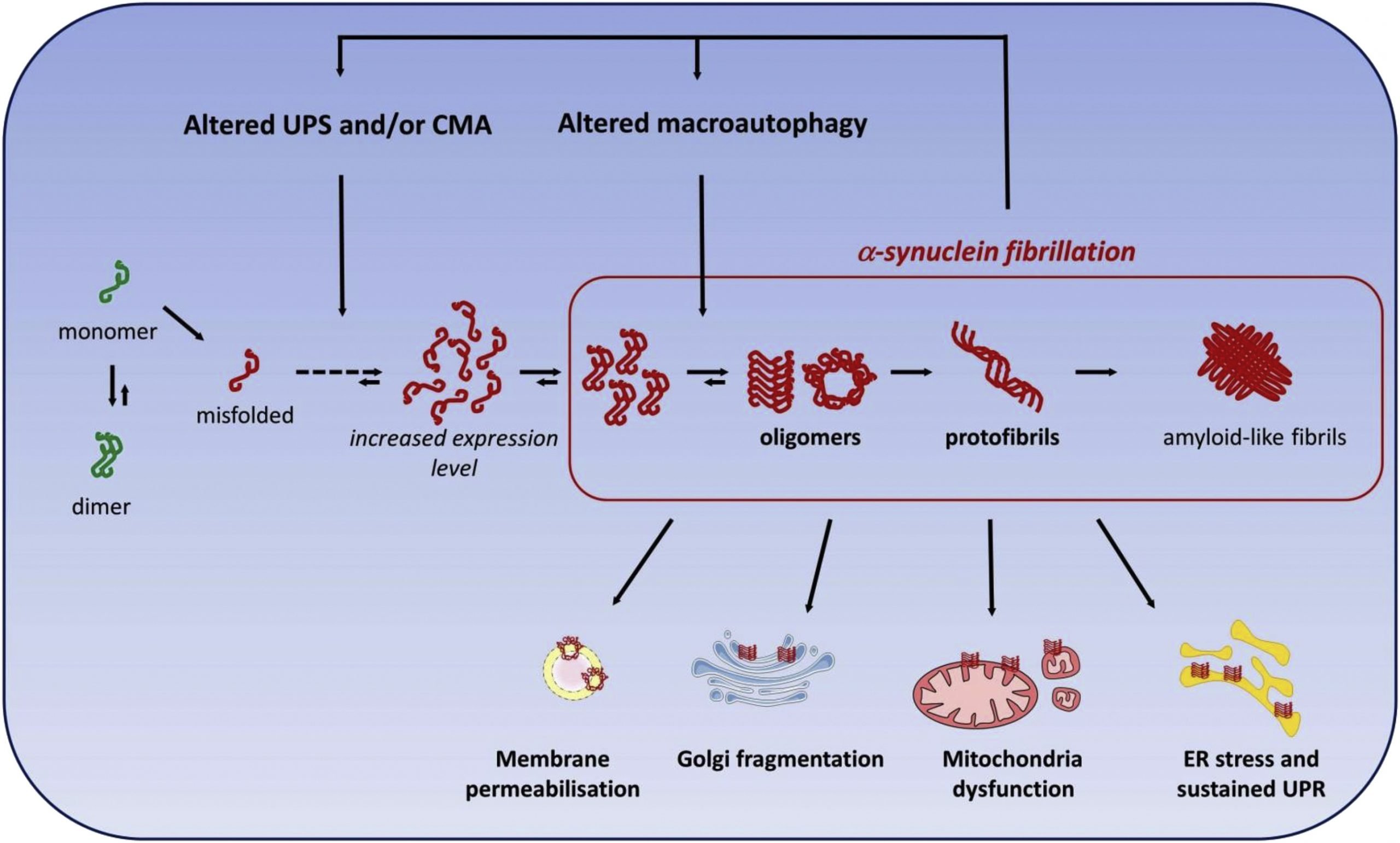
-Synuclein
PINK1 and Parkin
Other Causes Of Parkinsonism
“Parkinsonism” is the umbrella term used to describe the symptoms of tremors, muscle rigidity and slowness of movement.
Parkinson’s disease is the most common type of parkinsonism, but there are also some rarer types where a specific cause can be identified.
These include parkinsonism caused by:
- medication where symptoms develop after taking certain medications, such as some types of antipsychotic medication, and usually improve once the medication is stopped
- other progressive brain conditions such as progressive supranuclear palsy, multiple systems atrophy and corticobasal degeneration
- cerebrovascular disease where a series of small strokes cause several parts of the brain to die
You can read more about parkinsonism on the Parkinson’s UK website.
Page last reviewed: 30 April 2019 Next review due: 30 April 2022
Genetic Factors In Pd
Although PD was long considered to be sporadic in origin, monogenic Parkinsonism disorders are gaining growing importance in recent years. Genetic factors appear to be the main cause in about 510% of the PD patients . However, in both cases, the degeneration of nigrostriatal DA neurons remains a general overlapping characteristic . Studies have shown that around 13 genetic loci are involved in the rare forms of PD . Out of the 13, around 6 PARK loci genes have been identified and have been reported to carry mutations that are related to relatives who are affected by PD. Out of the six genes, four have similarly been shown to be involved in sporadic PD .
There is considerable evidence that, in addition to well-defined genetic mechanisms, environmental factors play a crucial role in PD pathogenesis. Nevertheless, the exact mechanism by which the environment could affect the genetic factors and contribute to PD development remains obscure. In recent years, epigenetic mechanisms such as DNA methylation, chromatin remodelling, and alterations in gene expression via non-coding RNAs are surging in importance as potential factors in the pathogenesis of PD.
Apoptosis In Parkinsons Disease
Apoptosis is the main mechanism of neuronal loss in Parkinsons disease, as evidenced by the identification of DNA fragmentation and apoptotic chromatin changes in dopaminergic neurons of Parkinsons disease patients in postmortem studies . In addition, the role of apoptosis in the pathogenesis of Parkinsons disease was confirmed in postmortem and in vitro studies that illustrated elevated activity of caspase-3 and increased expression of active caspase-3 in substantia nigra pars compacta . Furthermore, dopaminergic neuronal death is inhibited by overexpression of anti-apoptotic proteins, such as Bcl-2, in cell models of Parkinsons disease . Caspase inhibitors have also been shown to rescue neurons from death in cell models of Parkinsons disease, adding further support to the notion that apoptosis is the main mechanism of neuronal death in Parkinsons disease . Elevated levels of proapoptotic proteins, such as Bax, have also been seen in postmortem brain tissue from Parkinsons disease patients .
A number of mitochondrial toxins result in selective degeneration of dopaminergic nigral neurons through apoptosis, lending support to the idea that these neurons are particularly susceptible to mitochondrial dysfunction. These include 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine , rotenone, and 6-hydroxydopamine , which inhibit mitochondrial complex I causing mitochondrial dysfunction and generation of reactive oxygen species .
No Evidence For Transient Neurogenesis In The Snc
To test the possibility that the PCNA+ TH+ cells observed soon after MPTP intoxication were newborn neurons that failed to survive, we injected the retrograde tracer FluoroGold into the striatum, the major projection target of the SNc, labeling >90% of the nigral TH+ neurons . These mice were then acutely or subchronically treated with MPTP or NaCl and analyzed 24 h later. All identified PCNA+ TH+ cells were also FluoroGold-positive , demonstrating that they already existed before intoxication and were thus not newborn cells.
PCNA-expressing dopaminergic cells in the SNc of MPTP mice are not newborn neurons, but preexist. TH+ neurons in the SNc were labeled by striatal injection of the retrograde tracer FluoroGold 3 days before MPTP intoxication. Overview showing a cell in the SNc of a mouse 24 h after acute MPTP intoxication expressing both TH and PCNA . The presence of FluoroGold demonstrated that the cell existed before the lesion, ruling out the possibility that it was a stem cell-derived newborn dopaminergic cell. An enlarged detail from the boxed area in B shown as a confocal reconstruction in the xy, xz, and yz plane, to confirm the colocalization of TH, PCNA, and FG.
Research Story Tip: Common Mutation In Parkinsons Disease Increases Cell Calcium May Cause Brain Cell Death
Structure of the protein LRRK2. A study by Johns Hopkins Medicine shows that a mutation in the gene that makes this protein may be a factor in causing the brain cell death seen in Parkinsons disease. Credit: Public domain image
Johns Hopkins Medicine researchers have mapped out the cellular pathway that connects the most common genetic mutation associated with Parkinsons disease to brain cell death. In a new study, they show that the mutation initiates a biological pathway that could target brain cells most susceptible to the patterns of cell death leading to Parkinsons disease symptoms.
This deep dive into the molecular players in Parkinsons disease may provide some answers to its onset and progression, says Valina Dawson, Ph.D., professor of neurology at the Johns Hopkins University School of Medicine, and director of both the neuroregeneration and stem cell programs at the medical schools Institute for Cell Engineering.
The study, revealed that a mutation in the leucine-rich repeat kinase 2 gene shifts the balance of protein production within brain cells, allowing calcium to accumulate inside them. Though mutations in LRRK2 are the most common indicators of inherited Parkinsons disease, their functions within cells are not well understood.
This preference causes major problems down the line, because it may shift the levels of proteins whose precise regulation is essential for neurons to function and survive, explains Dawson.
Treatment Of Primary Culture
The medium was removed and replaced with fresh medium containing or not, either 1-methyl-4-phenyl-pyrididium , a metabolite of MPTP, 6OHDA or rotenone at different concentrations, for different times of incubation . Here, these three toxins were used to mimic in cell cultures in vitro cytopathic effects observed in the brains of PD-suffering patients. They were dissolved in the defined culture media mentioned above. Involvement of poly-ADP-ribose polymerase , a partner involved in necroptosis, was assessed using AG14361 , a specific inhibitor of PARP-1 .
Time For Caspase Activation
Once the mitochondrial membrane is permeabilized in response to an apoptotic stimulus, a number of molecules can freely diffuse from the mitochondria, including cytochrome c and procaspase-9. Cytochrome c induces polymerization of cytosolic Apaf-1, which is the adaptor protein of the mitochondrial pathway and is required for activation of caspase-9. Cytochrome cApaf-1 complex then binds procaspase-9 via a homologous death fold called CARD domain. In the next step, caspase-9 becomes activated and Apaf-1 and caspase-9 form the apoptosome complex. This apoptosome complex then plays a role in activation of caspase-3. Cells also produce heat shock proteins in response to stress, for example, Hsp90 and Hsp70. These directly associate with Apaf-1 and prevent caspases from being recruited to the apoptosome complex. Hsp70 can also bind AIF and block its proapoptotic activity .
and .
Caspases: The Key Molecular Players In Apoptosis
Caspases are a family of cysteine proteases that inactivate prosurvival proteins and activate proapoptotic proteins in an amplifying cascade. They cleave specific sites at aspartate residues of target substrates. Activation of caspases is often used as a marker of apoptosis.
There are two types of caspases: the ones involved in the initiation phase of apoptotic cell death called initiator caspases , and the ones involved in its execution called executioner caspases . The initiator caspases cleave and activate executioner caspases. The executioner caspases are then responsible for cleaving numerous substrates, which ultimately leads to cell death. At least hundreds of other substrates of caspase-3 have been identified.
Common Mutation In Parkinsons Disease Increases Cell Calcium May Cause Brain Cell Death
Johns Hopkins Medicine researchers have mapped out the cellular pathway that connects the most common genetic mutation associated with Parkinsons disease to brain cell death. In a new study, they show that the mutation initiates a biological pathway that could target brain cells most susceptible to the patterns of cell death leading to Parkinsons disease symptoms.
This deep dive into the molecular players in Parkinsons disease may provide some answers to its onset and progression, says Valina Dawson, Ph.D., professor of neurology at the Johns Hopkins University School of Medicine, and director of both the neuroregeneration and stem cell programs at the medical schools Institute for Cell Engineering.
The study, published Oct. 1, 2020, in the journal Cell Stem Cell, revealed that a mutation in the leucine-rich repeat kinase 2 gene shifts the balance of protein production within brain cells, allowing calcium to accumulate inside them. Though mutations in LRRK2 are the most common indicators of inherited Parkinsons disease, their functions within cells are not well understood.
This preference causes major problems down the line, because it may shift the levels of proteins whose precise regulation is essential for neurons to function and survive, explains Dawson.
Mapping out this progression of events is an important advancement in understanding the disease and provides more information on how Parkinsons disease may initially arise, says Dawson.
Apoptosis: Type I Cell Death
Apoptosis is an evolutionarily well-conserved molecular process. It is also the most common and the best-described form of PCD. Although phenotypes of apoptotic cells may differ, typical morphological features include shrinking of the cell, membrane blebbing, compartmentalization, chromatin condensation, and DNA fragmentation. The advantage of this highly regulated process over classical necrosis is that during apoptosis the membrane integrity remains intact with the contents of the dying cell enclosed within apoptotic bodies, without releasing them into an extracellular space. The dying cell expresses cell surface markers that target it for recognition and phagocytosis by adjacent cells, without activating an inflammatory response. Therefore, this type of cell death may be regarded as cleaner and preferred, provided the cell has enough energy to go through with the death program.
Numerous assays have been developed to detect apoptosis, including ultrastructural analysis assessing cellular morphology, testing for DNA fragmentation, detection of caspases, their cleaved substrates, or regulators of apoptosis, or methods assessing mitochondrial function .
Overexpression of wild-type PINK1 has a prosurvival effect by inhibiting opening of the mitochondrial PTP . However, mutant PINK1 or depletion of PINK1 sensitizes to apoptotic death.
Damage To Dopaminergic Neurons By Oxidative Stress In Parkinson’s Disease

This article is mentioned in:
Abstract
1. Introduction
2. Mitochondrial complex inhibition and ROSproduction
3. Vulnerability of dopaminergic neurons tooxidative stress
4. Reactive oxygen species and mitochondrialdysfunction
5. Oxidative stress and the opening of themPTP
6. Oxidative stress andneuroinflammation
7. Damage to nucleic acids by oxidativestress
8. Conclusion
Abbreviations:
Acknowledgments
Notes
References
1
Gain Of Function Mutations In Parkinsons Disease
Autosomal dominant PD is caused by mutations in -synuclein and LRRK2 , indicating gain-offunction mutations for both proteins. Interestingly, -synuclein is a major component of Lewy bodies and Lewy neurites, which constitute two of the main pathological hallmarks of PD. This observation demonstrates how familial and sporadic forms of PD are molecularly interrelated, emphasizing the notion that insight into the pathogenesis of familial PD should critically advance our knowledge of sporadic PD . This, in turn, implies that drug therapies for familial PD should be applicable to the PD population at large, rather than be confined to a few rare cases.
LRRK2 is the other protein mutated in an expected gain-of-function manner. Similarly to the case with -synuclein, there is evidence that knowledge about pathogenesis of familial PD will translate effectively into understanding sporadic PD. As LRRK2 is very commonly mutated in sporadic cases, with estimates ranging up to 4%, there is great enthusiasm in the research community that understanding LRRK2 function and contribution to PD pathogenesis will benefit a wide spectrum of PD patients .
Primary Culture Of Mesencephalic Neurons
The collection of embryos was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and followed current European Union regulations and was supervised and approved by the local direction of the veterinary services of the Bouches-du-Rhône .
Pregnant female rats of 15 days gestation were killed using a deep anesthesia with CO2 chamber followed by cervical dislocation.
Rat dopaminergic neurons were prepared and cultured as previously described by Visanji and colleagues . Briefly, the midbrains obtained from 15-day old rat embryos were dissected under a stereo zoom binocular microscope. The embryonic midbrains were removed and placed in ice-cold L15 medium of Leibovitz containing 2% of penicillin and streptomycin solution and 1% of bovine serum albumin . The ventral portion of the mesencephalic flexure, a region of the developing brain rich in dopaminergic neurons, was used for the cell preparations.
Stem Cells As A Source Of Healthy Neurons To Treat Pd
There is strong evidence to suggest that replacing the dopamine-producing neurons that die in the midbrain could significantly help PD patients. Implanting newly generated dopamine-releasing cells in the brain of patients with PD at the site where dopamine normally signals may give a clinical response that is equivalent to that seen with dopamine drugs, with the advantage that the grafted neurons would release dopamine in a physiological way at the site where it is needed, and by so doing should avoid the side effects seen with the drugs.
Beginning in the 1980s, attempts have been made to repair the PD brain using dopamine-producing cells, the most successful attempts being transplants of dopamine cells from fetal tissue. These cells, when grafted into patients with PD, can survive long-term in large numbers, release dopamine, integrate and function in the host brain, and significantly improve PD for years. This approach, while demonstrating proof-of-principle, however, was found to have technical, logistical, and quality control issues, mostly due to the fetal cell sources: inconsistent or poorly-defined cells, undesirable side effects in some trials, and policy issues in the U.S. around the use of fetal cells. Therefore, a more standardized source of dopamine cells is needed.
Dopaminergic neurons derived from human ES cells. From Wang et al., 2018 Stem Cell Reports.
