Sites Of Deep Brain Stimulation And Symptom Control
While both subthalamic nucleus and globus pallidus internus stimulation help improve the motor symptoms of Parkinsons disease, studies have found a few differences.
DBS of the third target, the ventral intermediate nucleus, can be beneficial for controlling tremors but does not work as well at addressing the other motor symptoms of Parkinsons disease.
In a Canadian study, targeting the subthalamic nucleus allowed people to reduce the doses of their medications to a greater degree, while targeting the globus pallidus internus was more effective for abnormal movements .
In another study, STN deep brain stimulation also led to a greater reduction in medication dosages. However, GPi stimulation resulted in greater improvement in quality of life, and also appeared to help with the fluency of speech and depression symptoms.
Side effects of DBS can sometimes include subtle cognitive changes . A different study compared these effects with regard to these different areas.
GPi showed smaller neurocognitive declines than STN, though the effects were small with both. On a positive note, both procedures seemed to reduce symptoms of depression following surgery.
What Happens Before Deep Brain Stimulation
Before this procedure, your healthcare provider will discuss the advantages and disadvantages of having a DBS device implanted. Theyll also explain the possible risks that come with this surgery. Theyll also verify that you can have this surgery, which can involve other imaging scans or lab tests to look for any reasons you may not be able to have the procedure.
If you still decide you want to have the DBS implanted, your provider will then have you get detailed magnetic resonance imaging and computed tomography scans of your brain. These scans will help your provider decide which location is the best place to place the wires for the DBS.
Before the procedure, your provider will also talk to you about the following:
Clinical Experience With Deep Brain Stimulation
The advent of modern DBS led to a major change in the therapeutic armamentarium for movement disorders. DBS rapidly overtook lesioning as the surgical treatment of choice for refractory movement disorders due to a number advantages: it is nondestructive and several stimulation parameters, including the location, size, intensity, and the shape of the stimulating current field can be adjusted following surgical implantation. These properties allow clinicians to program the DBS device in such a way as to maximize motor benefits while minimizing side effects, most of which are caused by the inadvertent stimulation of structures adjacent to the intended target. Perhaps most importantly for patients with PD, DBS has a lower reported complication rate when used bilaterally .
Since the first application of DBS for PD in 1993, several thousand patients worldwide have undergone surgical implantation. While many studies have reported the benefits and durability of this therapy , six large-scale, randomized, controlled clinical trials have been performed . Given the pervasive nature of this disease, the end points of these trials have appropriately included quality of life measures, the severity of motor symptoms in the medication off state, and time spent in the on state without troublesome motor symptoms .
You May Like: Is It Parkinson’s Or Something Else
What Is Deep Brain Stimulation How Does It Work
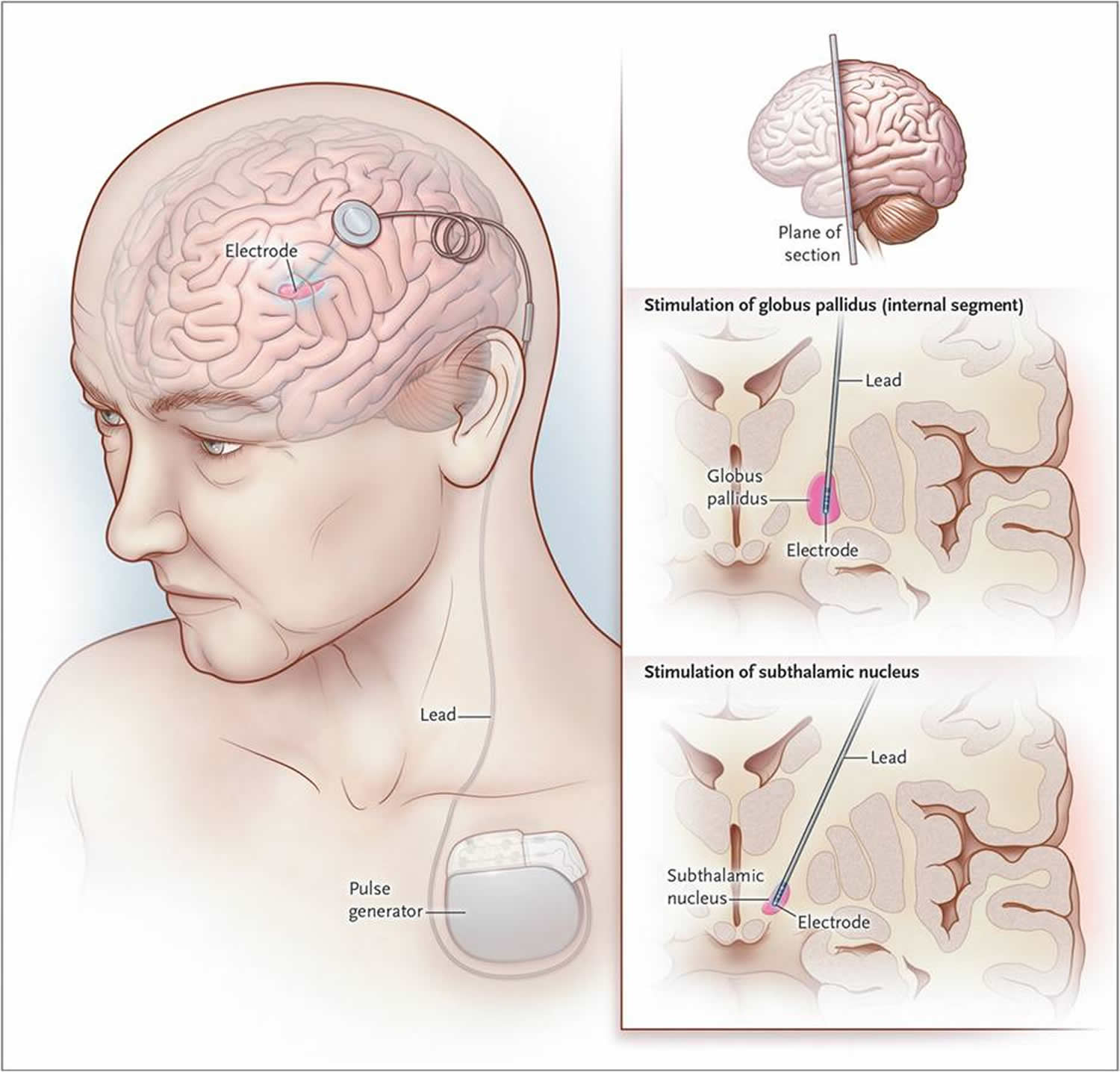
Deep brain stimulation is a surgical procedure that involves the placement of electrodes in certain areas of the brain, such as the subthalamic nucleus or basal ganglia. The electrode placement is done by a surgeon. It helps to deliver electrical stimulation in the form of bilateral deep brain stimulation.
The amount of stimulation the DBS electrodes provide is controlled by a cardiac pacemaker-like device placed in the upper chest. The DBS system also includes an extension wire or wires connecting the implant device in the chest to the DBS lead or electrodes in the brain.
As mentioned above, abnormal electrical impulses from nerve cells cause severe motor fluctuations in Parkinsons patients. DBS electrodes deliver continuous electrical pulses to regulate these abnormal electrical signals generated by the brain cells, thus providing relief from motor symptoms to Parkinsons patients.
How Effective Is Dbs For Parkinson’s
Deep brain stimulation surgery can lead to a significant improvement in motor symptoms such as slowness, stiffness, tremors, and dyskinesias. It can also result in lower medication doses in some patients. However, the DBS procedure does not work as well for symptoms such as freezing of gait, imbalance, and other non-motor symptoms. Deep brain stimulation can actually worsen thinking and memory problems in some patients. Therefore, DBS is usually not recommended for people with dementia symptoms.
Don’t Miss: Does Parkinson’s Affect Your Brain
Can I Keep Using Other Treatments
In most cases, yes, but always consult your doctor to ensure that this is appropriate for you or your loved one. With your doctors approval, the Exopulse Mollii Suit should ideally be used in combination with physiotherapy, training, or physical activity. Discuss the Suit with your doctor if you or your loved one are currently receiving botulinum toxin injections or have been prescribed oral medication for spasticity. Do not use the Suit if you or your loved one have an implanted medical device .
You May Like: Boxing And Parkinsons Disease
Major Depression And Obsessive
DBS has been used in a small number of clinical trials to treat people with severe treatment-resistant depression . A number of neuroanatomical targets have been used for DBS for TRD including the subgenual cingulate gyrus, posterior gyrus rectus,nucleus accumbens, ventral capsule/ventral striatum, inferior thalamic peduncle, and the lateral habenula. A recently proposed target of DBS intervention in depression is the superolateral branch of the medial forebrain bundle its stimulation lead to surprisingly rapid antidepressant effects.
The small numbers in the early trials of DBS for TRD currently limit the selection of an optimal neuroanatomical target. Evidence is insufficient to support DBS as a therapeutic modality for depression however, the procedure may be an effective treatment modality in the future. In fact, beneficial results have been documented in the neurosurgical literature, including a few instances in which people who were deeply depressed were provided with portable stimulators for self treatment.
DBS for TRD can be as effective as antidepressants and can have good response and remission rates, but adverse effects and safety must be more fully evaluated. Common side effects include “wound infection, perioperative headache, and worsening/irritable mood increased suicidality”.
Also Check: What Type Of Disease Is Parkinson’s
Who Can Have Dbs Surgery
If you have worsening Parkinsons symptoms and your medications are not effective enough, then you may be recommended to have the DBS procedure done.
However, DBS will not be recommended in some instances.
These include scenarios where the Parkinsons patient has severe depression, advanced forms of dementia, or have symptoms that are not typically associated with Parkinsons disease.
How Does Deep Brain Stimulation For Parkinsons Work
Deep brain stimulation works by modifying abnormal electrical activity in the brain. It was first approved for Parkinsons tremors in 1997 and has become an established treatment to control additional motor symptoms of Parkinsons disease.
DBS involves three main components:
- Leads: Leads are implanted in the brain in a region responsible for motor activity.
- Implantable pulse generator : A separate procedure is performed to implant a battery-operated device in the chest or in the abdomen. An IPG is similar to a pacemaker for the heart and has been coined by some as a pacemaker for the brain.
- Extension: A thin, insulated wire is passed beneath the skin between the leads and implantable pulse generator to deliver the electrical stimulation from the pulse generator to the leads.
The target area in the brain is first identified by magnetic resonance imaging or computed tomography . Then, the leads are placed via small holes that a surgeon drills in the skull.
This is considered a minimally invasive surgery that is done in the operating room with local anesthesia. It usually requires an overnight stay.
The IPG is inserted in a separate surgical procedure in the operating room roughly a week later.
After a few weeks, a neurologist begins to program the unit. This process can take several additional weeks to months. When this is completed, people are able to manage the device with a handheld remote control.
Also Check: What Is The Difference Between Alzheimer’s Dementia And Parkinson’s
Life After Dbs Surgery
Once the neurotransmitter has been programmed, you are given a handheld controller to make adjustments.
With the controller, you can turn the simulator on or off, select the signal strength, and move across different program types.
If your DBS neurotransmitter has a rechargeable battery, then it will take about two hours for the device to recharge completely.
Make sure to carry your Implanted Device Identification card if you are traveling by air, as Airport Security will detect the device.
Resources For More Information
- Surgical option a potential life-changer for patients with OCD: Read and watch Erins story as she, a lively 21-year-old woman, fought her battle with OCD. This article explores how deep brain stimulation gave Erin her life back. The procedure was the first of its kind performed at Albany Medical Center the only facility offering this treatment between New York and Boston. In Erins own words, “Now, I can be who I really am and tell people my story and hopefully inspire people and help people along the way.
- Karen and Jims Story: A Shared Journey of Life, Love and DBS: Read about Karen and Jim. They were each diagnosed with Parkinsons before they met. Follow them on their journey as they fall in love after meeting each other from an online support group. See how they embraced each other and DBS.
- Kays Story A Parkinsons Disease Patient: Read about Kay, a 68-year-old woman suffering from Parkinsons disease. The article and video explore how DBS helped her regain her life. In Kays own words, Its like I had been turned on again. It was like a miracle.
Don’t Miss: Did Katharine Hepburn Have Parkinson’s Disease
Why Is Dbs Used
There are billions of neurons in each human brain, and these cells communicate with each other using electrical and chemical signals. Several brain conditions can make neurons in different parts of your brain less active. When that happens, those parts of your brain dont work as well. Depending on the part of the brain affected, you can have disruptions in the abilities controlled in that area.
DBS uses an artificial electrical current to make those neurons more active, which can help with the symptoms of several different brain conditions. However, researchers still dont know exactly how or why this works.
Deep Brain Stimulation Surgery
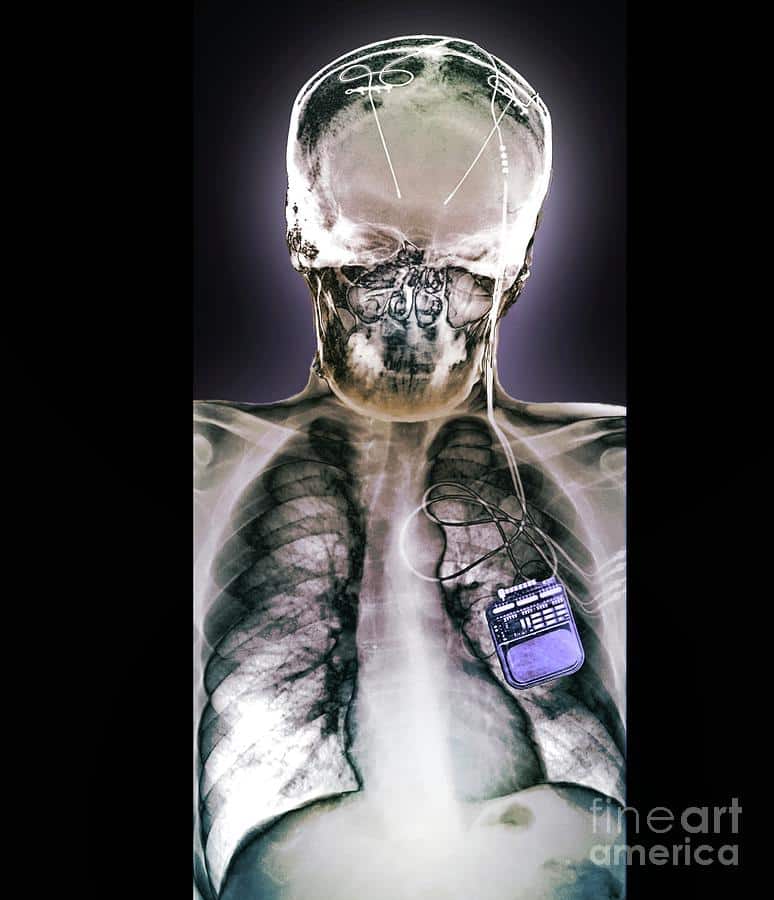
A team of experts, including a movement disorder specialist and a brain surgeon, conducts an extensive assessment when considering DBS for someone. They review your medications and symptoms, examine you when you’re on and off Parkinson’s medication, and take brain imaging scans. They also may do detailed memory/thinking testing to detect any problems that could worsen with DBS. If your doctors do recommend you for DBS and you are considering the surgery, discuss with your care team the potential benefits as each person’s experience is unique. It’s also critical to discuss the potential surgical risks, including bleeding, stroke and infection.
In DBS surgery, the surgeon places thin wires called electrodes into one or both sides of the brain, in specific areas that control movement. Usually you remain awake during surgery so you can answer questions and perform certain tasks to make sure the electrodes are positioned correctly. Some medical centers now use brain imaging to guide the electrodes to the right spot while a person is asleep. Each method has its pros and cons and may not be suitable for everyone or available everywhere.
Once the electrodes are in place, the surgeon connects them to a battery-operated device , which usually is placed under the skin below the collarbone. This device, called a neurostimulator, delivers continuous electrical pulses through the electrodes.
Also Check: Parkinson’s Disease Causes Symptoms And Treatment Ppt
Conflict Of Interest Statement
UCSF has submitted two pending/provisional patents related to use of particular brain signals for closed loop DBS. Drs. NS and CH are included as co-inventors on these patents.
References
Little, S., Brown, P. 2014. The functional role of beta oscillations in Parkinsons disease. Parkinsonism Relat. Disord. 20:S44S48. doi:10.1016/S1353-802070013-0
de Hemptinne, C., Swann, N. C., Ostrem, J. L., Ryapolova-Webb, E. S., San Luciano, M., Galifianakis, N. B., et al. 2015. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinsons disease. Nat Neurosci. 18:779786. doi:10.1038/nn.3997
Little, S., Pogosyan, A., Neal, S., Zavala, B., Zrinzo, L., Hariz, M., et al. 2013. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 74:449457. doi:10.1002/ana.23951
Who Is A Candidate For Deep Brain Stimulation
DBS is more than just a surgical procedure. It involves a series of evaluations, procedures, and consultations before and after the actual operation, so people interested in being treated with DBS should be prepared to commit time to the process.
For example, those who do not live close to a medical center that offers DBS surgery may need to spend significant time traveling back and forth to appointments.
The procedure, as well as the pre-operative evaluation and post-operative follow-up, can be expensive depending on the persons insurance coverage. DBS surgery is an FDA-approved treatment for Parkinsons disease, and Medicare and most private insurers cover the procedure, but the extent of coverage will depend on each persons individual policy.
Prospective patients should have realistic expectations about DBS results. Although DBS can improve movement symptoms of Parkinsons disease and greatly improve quality of life in properly selected patients, it is not likely to return anyone to perfect health.
You May Like: Can A Person With Parkinson’s Disease Drive A Car
How Deep Brain Stimulation Works
Exactly how DBS works is not completely understood, but many experts believe it regulates abnormal electrical signaling patterns in the brain. To control normal movement and other functions, brain cells communicate with each other using electrical signals. In Parkinson’s disease, these signals become irregular and uncoordinated, which leads to motor symptoms. DBS may interrupt the irregular signaling patterns so cells can communicate more smoothly and symptoms lessen.
How You Can Control Parkinsons Disease Symptoms With Deep Brain Stimulation
There is no cure for Parkinsons disease, but neurological specialists can help patients control the tremors and other symptoms that patients experience through a procedure called deep brain stimulation .
Parkinsons disease is a brain disorder that results in shaking and tremors, and difficulty with walking, movement and overall coordination. The disorder is associated with damage to a part of the brain that involves movement.
In many cases of Parkinsons, symptoms can be managed and virtually eliminated through DBS.
Read Also: Advances In Parkinson’s Treatment
Mechanism Of Action Of Dbs
Current hypotheses on the action mechanism of DBS include depolarization blockade , synaptic inhibition , synaptic depression , stimulation-induced disruption of pathological network activity , and stimulation of afferent axons projecting to the STN . Depolarization blockade and synaptic inhibition are likely to explain the similarity between the therapeutic benefit of DBS and lesional surgery. Recordings of decreased somatic activation in the stimulated nucleus favor these hypotheses . However, the increased output of projection neurons does not seem to be mediated by these phenomena . Another and currently favored hypothesis is that DBS overrides abnormal spike train patterns by an unphysiological, high-frequency pattern, and thereby masks pathological signals, which cause dysfunction of the remaining elements of the basal ganglia-thalamo-cortical and brainstem motor loop . The exact nature of the abnormal signals and the interaction between stimulation-induced neuronal responses and intrinsic brain activity remains elusive, but abnormalities of the firing rate and pattern of basal-ganglia neurons, changes in oscillatory activity and excessive synchronization at multiple levels of the motor loop have been proposed as pathophysiological correlates of motor symptoms in PD .
Data Collection And Outcomes
A physiotherapist and a medical doctor blinded to the frequency of stimulation conducted the assessments before, during, and after each intervention session at day 1, 2, and 3.
Data regarding age, gender, independence in walking , and independence in mobility and personal care were collected. In addition, information about stroke type , time to inclusion from stroke onset and paretic side were obtained. Sensorimotor function including voluntary and passive movement, tactile sensibility to light touch and proprioception and pain were rated according to the FuglMeyer scale for upper and lower extremity , yielding a maximum of 126 and 86 points, respectively, where a lower score indicates more severe impairment .
Measure of Spasticity
Changes in spasticity during and after the use of the EXOPULSE Mollii suit represented the main outcome and were investigated with the NeuroFlexor hand and foot modules. Additionally, surface electromyography was used to evaluate reflex muscle activity in the spastic muscles in the upper and lower limb.
Additional Clinical Measure
Although spasticity is neural in origin, concomitant changes in the soft tissue occur generally after stroke. Thus, limitation in joint movement is an important component of evaluation. Passive and active range of motion of wrist and ankle were measured by the use of a goniometer before and after stimulation, and a change in ROM measurements > 5° was considered clinically significant .
You May Like: Latuda And Parkinson’s Disease
A Potential Parkinsons Breakthrough
Scientists at the University of Gothenburgs Sahlgrenska Academy in Sweden have identified a potential breakthrough in Parkinsons research. They have developed a wearable device that generates electronic noise, which can help patients improve their motor skills and balance. Even though the development of the device and research on the impact it can have on patients is still in its infancy, its promising news.
The research was led by associate professor Filip Bergquist. This is really not a very complicated device. It is a current device which is very similar to the ones that people use for pain relief with electrical stimulation of muscles and nerves, whats called TENS , Bergquist said to Reuters.
The difference is that we use a particular current profile which you can stimulate the balance organs with without creating a balance disturbance. So you do not get the impression that the world is moving or that you are moving, you actually do not feel anything.
The portable, pocket-sized device stimulates the organs associated with balance through patches attached to a patients head, helping to mitigate the effects of insufficient dopamine production in the brain. Currently, patients are treated with the drug levodopa, which often leads to involuntary movements as a side effect.
A longer-term study is in the works, which would involve patients using the device at home. If progress is made, the device could be offered as a treatment option within the next five years.
