Hidden Signatures Of Parkinsons Disease Revealed By Nyscfs Automation Technology And Artificial Intelligence
The Context: The vast majority of drugs fail clinical trials, largely because there is still much we do not understand about the underpinnings of complex diseases like Parkinsons or Alzheimers, and most drugs are discovered using animal models that do not adequately capture how a disease manifests in humans. We need a better system for discovering exactly how our cells react to disease so that we can figure out how to treat them.
The Study: A new platformdeveloped by NYSCF scientistsintegrates robotic systems for studying patient cells with artificial intelligence methods for image analysis to identify cellular features of disease. Their collaborative study with Google Research, in Nature Communications, examined more than 6 million images to successfully identify new hallmarks of Parkinsons patient skin cells in comparison to healthy controls.
The Importance: This platform allows scientists to study diseases like Parkinsons at an unprecedented cellular level, identifying features of disease invisible to the human eye that can be targeted by new or existing drugs. The system could also distinguish subgroups of Parkinsons patients with varying features, which is key groundwork for advancing personalized medicine.
Improved Monitoring Of Pd
One system which is FDA cleared and is available on the market for monitoring of PD patients between office visits is known as the Personal KinetigraphTM . A patient wears the watch-like sensor for 6-10 days in anticipation of an office visit. The sensor collects data which is then interpreted through the PKG TM algorithms to measure bradykinesia and dyskinesia throughout the time period that the watch is worn. The PKG TM also has the ability to alert a patient to take a medication dose and allows the patient to record whether the dose was taken. After the data is collected, the patient mails the watch in and the data is downloaded and sent to the patients physician so that the physician has the information for the patient visit. The information can then complement what the patient and care partner say about their medication responses at home. A poster at the International Congress demonstrated that when PKG TM is in use, it often influences and informs the decision to change medications.
Practical Limitations In User Engagement
Software and hardware components of wearable systems are often not as user friendly or compelling to adopt as they should be.44 Currently, patient and caregiver engagement with wearable and mobile technology is modest, as shown by a recent study demonstrating that 32% of users stop using wearables after 6 months, and 50% after just over a year.45 Similarly, there is a high dropout rate amongst smartphone apps users: 26% of apps are used only once and 74% of apps are not used more than 10 times.45, 46 Lack of motivation to use wearables/self-monitoring systems should not be underestimated, particularly in the absence of meaningful feedback provided to their users. Preliminary evidence suggests that patient empowerment and their inclusion as active players in the development of research activities may favorably impact compliance.47 Research is needed to determine the characteristics of wearable systems for long-term monitoring of motor and non-motor symptoms that would be acceptable to patients. In particular, we need to ascertain the number of sensors needed to accurately monitor PD symptoms without negatively affecting compliance in a clinical context.
Read Also: Is A Stiff Neck A Sign Of Parkinson’s
New Ultrasound Technology For Treating Parkinsons Disease Patients Under Clinical Trial
Researchers from the University of Maryland Medicine and from its Center for Metabolic Imaging and Image-Guided Therapeutics are conducting the first clinical trial with ultrasound waves to treat Parkinsons disease patients. Using magnetic resonance imaging , they guide ultrasound waves through the intact skin and skull to a deep brain region, the globus pallidus. This structure regulates voluntary movement and can be targeted by medication and surgery to treat motor symptoms of tremor, rigidity and dyskinesia in patients with PD.
Levodopa is the current treatment for PD and can temporarily diminish motor symptomatology. However, in the long term, levodopa side-effects include involuntary movements called dyskinesias. Patients with advanced PD whose symptoms are not treatable by medication may undergo a surgical procedure known as deep brain stimulation. One of the brain regions stimulated by implanted electrodes is the globus pallidus though this surgery has associated risks.
This new procedure lasts two to four hours with patients awake and lying on an MRI scanner with a head-immobilizing frame fitted with a transducer helmet. Ultrasonic energy is targeted through the skull to the globus pallidus of the brain, and images acquired in real-time. This allows the physicians to monitor the area being targeted and to make adjustments if necessary.
What Is Parkinsons Disease
Parkinsons disease is a progressive brain disorder that causes shaking and muscle stiffness, and slows movement. It develops when neurons in a particular part of the brain stop working properly and are lost over time. These neurons produce an important chemical called dopamine. Dopamine is used by the brain to send messages across brain areas to help control movement. Eventually, the brain cannot make enough dopamine to control the movement properly.1,2
Dont Miss: Parkinsons Disease Dementia Prognosis
Also Check: Best Vitamins For Parkinson’s
Advanced And Future Treatments For Parkinsons
While theres no cure for Parkinsons disease, recent research has led to improved treatments.
Scientists and doctors are working together to find a treatment or prevention technique. Research is also seeking to understand who is more likely to develop the disease. In addition, scientists are studying the genetic and environmental factors that increase the chance of a diagnosis.
Here are the latest treatments for this progressive neurological disorder.
In 2002, the FDA approved deep brain stimulation as a treatment for Parkinsons disease. But advances in DBS were limited because only one company was approved to make the device used for the treatment.
In June 2015, the FDA approved the
Innovative Gel Offers New Hope To Defeat Parkinsons Disease
When we introduced the gel technology with the stem cells we saw huge improvement in the animals coordinated paw movement and overall motor function recovery.
Researchers from The Australian National University , in collaboration with The Florey Institute of Neuroscience and Mental Health, have developed a new type of hydrogel that could radically transform how we treat Parkinsons disease. The gel also offers hope for patients who have suffered from other neurological conditions such as strokes.
The new material is made from natural amino acids the building blocks of proteins and acts as a gateway to facilitate the safe transfer of stem cells into the brain and restore damaged tissue by releasing a growth-enabling protein called GDNF.
The research has been published in the journal Advanced Functional Materials.
Don’t Miss: Parkinson’s Disease Slow Progression
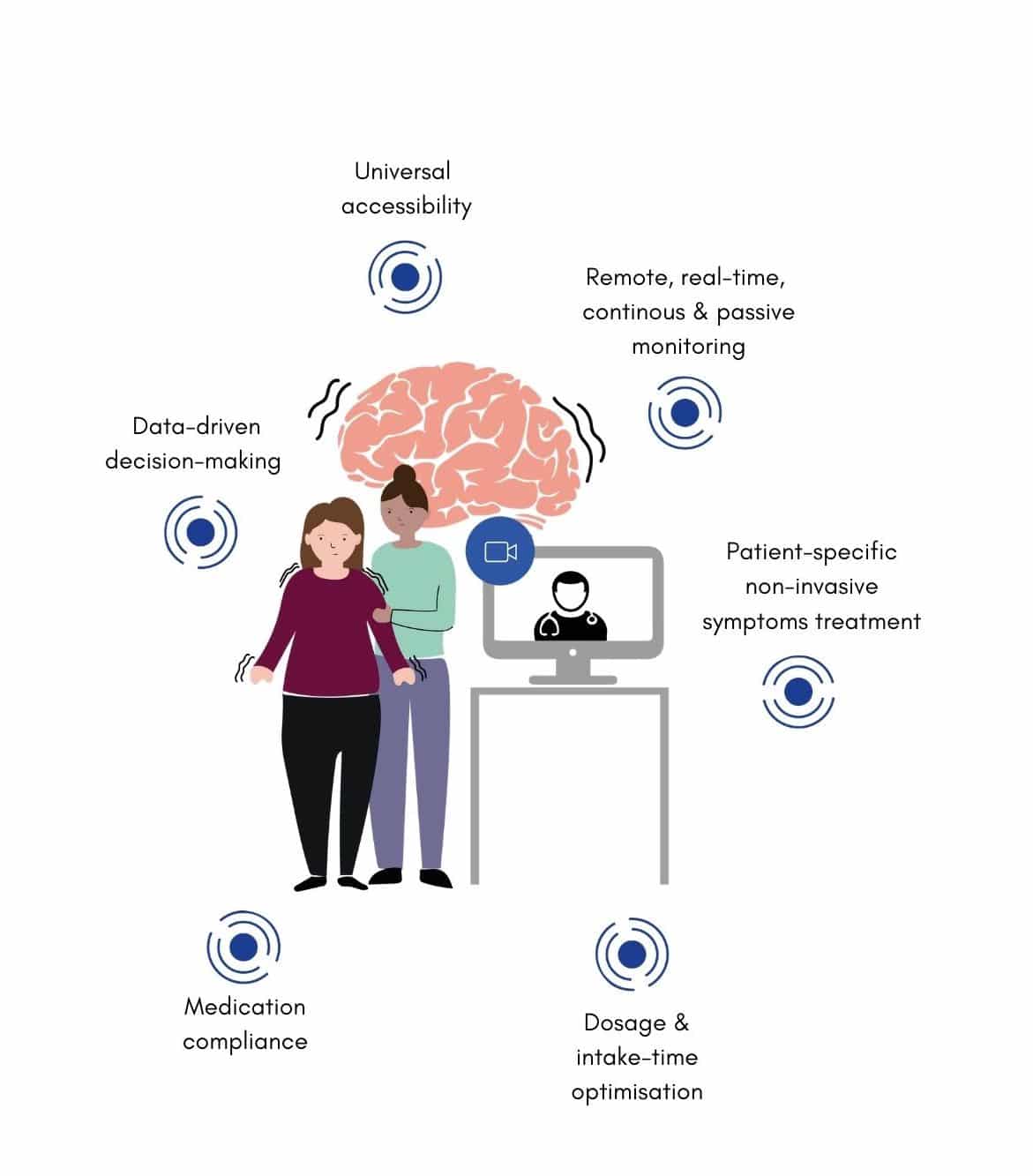
New Technology Allows Doctors To Treat Parkinsons Disease Patients Remotely
Deep Brain Stimulation – AdventHealth TV Story
At just 35 years old, Carlos Paredes got a stunning diagnosis: He had Parkinsons disease.
I did not want to believe that I had Parkinsons at my age, he said. At that time, I was about to get married, and I was ready to start a family. So, it was hard for me.
Paredes initially noticed tremors in one of his fingers. Over the years the uncontrollable movements spread to all his fingers, and eventually to both arms. The tremors were so severe that he struggled with simple tasks such as drinking from a cup, writing and brushing his teeth.
My movements increased to the point I could not hold a plate for dinner, I could not play with my daughter or shave, he said.
His family advised him to see a neurologist, who diagnosed him with Parkinsons disease a progressive condition that causes tremors, slowness and balance issues. After years of taking up to 16 pills daily without much improvement, his mother suggested he see an expert in brain surgery.
In consultation with his medical team, which included neurosurgeon Dr. Chandan Reddy at AdventHealth Celebration and Dr. Mitesh Lotia, movement disorder neurologist and medical director of the movement disorder program at AdventHealth, it was determined that Paredes was an ideal candidate for deep brain stimulation surgery.
“My life has improved 90% because I do not have the movements anymore.”
Leveraging Medical Devices For Therapy
When PD patients do not respond well to medication or experience a decline in medication effectiveness over time, technology offers a different method of treatment. Deep brain stimulation is a well-established therapy for motor symptoms and involves the implantation of a small device that sends electrical signals to targeted areas of the brain. This therapy can improve patient quality of life by treating motor symptoms of the disease, including tremor, stiffness and slowed movement.
Advances in DBS technology have focused on making these electrical signals more adaptive to fluctuations in symptoms or even brain signals that the device can detect, rather than sending a constant signal predetermined by the patients doctor. As the study of DBS grows, more information will be discovered about this technologys ability to offer patients better treatment options that can adjust to their changing needs.
Further, the use of telehealth systems can help physicians to remotely manage patients undergoing DBS at home , both making access to healthcare easier for patients and illustrating a method for clinical researchers to gather data in real time and generate endpoint data.
Recommended Reading: Is Essential Tremor Related To Parkinson
Limitations Of Sensors Used To Monitor Motor Symptoms
Biomechanical sensors such as accelerometers, gyroscopes, and magnetometers are well suited for the detection of tremor, bradykinesia, gait impairment, and motor complications, such as dyskinesia. However, data collected in the home and community settings using these sensors do not always provide sufficient information to achieve a reliable clinical assessment of motor symptoms. For instance, it is difficult to infer from the sensor data alone if slowness of movement can be used as a proxy of bradykinesia, or is the result of fatigue or other factors related to the context in which a motor task is performed . Also, the resolution of biomechanical sensors is quite restricted to the anatomical area on which they are applied, which may yield low quantitative agreement with the wider range of motor disability, quality of life, and other measurable patient-relevant endpoints.36, 37
Integration Into Medical Care And Reimbursement
Payers do not yet provide reimbursement for medical services provided by TOMs and companion apps. This limits the rate of innovation and the opportunities for integration of TOMs into medical care. Establishing reimbursement mechanisms will require demonstration that, along with the enhancements in diagnostics and therapeutics, TOMs can be integrated in quality control concepts, help reduce costs and time, as well as improve patientsâ quality of life while guarding against privacy concerns. Quantifying clinical benefits of interventions using TOMs is anticipated to become increasingly important in healthcare as the allocation of resources is expected to be tied to objective outcome measures.
Also Check: Can Medications Cause Parkinson’s Disease
Funded By Leading Charities
Parkinsons UK and The Michael J. Fox Foundation , two leading charities have raised £1.5m to fund the phase 2 clinical trial, which is being sponsored by the biopharmaceutical company Neurolixis.
Dr Arthur Roach, Director of Research at Parkinsons UK, said: Were pleased to be supporting this study which aims to deliver a treatment that is desperately needed by many people living with Parkinsons. Its great that recruitment is now underway as this milestone brings us one step closer to results which could reveal an important new therapy for the millions living with this condition around the world.
Caveat Emptor: Why Measure At All
The often-implied assumption that the sole existence of a PD symptom justifies its measurement and that all PD-related phenomena should be measured must be dispelled. A measure is justified if it enhances our understanding of a complex disease or aids in testing or delivering a therapy. The use of measurements to improve therapy is filled with rich examples from other branches of medicine . It should be remembered that every qualitative clinical assessment is a form of measurement and that the use of quantitative measures carries potential for improving the decision-making process as to the need and dose of therapy. Implicit however is that what is being measured represents a therapeutic target and hence the measurement must be relevant to the treatment question .
Don’t Miss: Can Stem Cells Cure Parkinson’s
Additional Note: Keep An Eye Out For
Cue, by Nanotech
What we liked about the progress of CUE :
We’re including this to give you something to think about in the future, or to join their waitlist for updates. The product is well designed, has a strong team, reputable support, good early customer experiences, and shares trial results.
CUE a non-invasive wearable device for Parkinsons, utilizes pulsed cueing and focused vibrotactile stimulation to reduce symptoms of slowness and stiffness resulting in improved movement. It delivers a gentle vibratory stimulus to the peripheral nervous system, producing a characteristic cortical response that eases the typical stiffness and slowness symptoms associated with Parkinsons.
Users of CUE for Parkinsons report feeling that movement is better controlled and they can move smoother, easier and faster whilst wearing the device.
The product is still in testing. Follow the link to learn more, and read the science, customer testimonials, and early research indications. Estimates indicate an average of 16% improvement in movement. Additional features include tracking symptoms and medication assistance.
Note We will continue to monitor the progress of this product. Please do your own research.
Can I Benefit From Assistive Technology
Answer the following questions to see if assistive technology may help you:
- Can I communicate effectively and be understood by others?
- Can I use my computer to read or type as effectively as in the past?
- Do tremors impact my ability to type?
- Do I have difficulty using a touch screen?
- Am I having more difficulty remembering passwords?
- Am I able to effectively control electronics within the home environment?
- Have I experienced falls or loss of balance?
Also Check: What Happens To The Basal Ganglia In Parkinson’s
Dedicated Parkinson’s Data Collection Wearable For Health Providers With Patients
PKG Kinetics Watch – Global Kinetics
What we like about the PKG:
-
A dedicated wrist-worn device, prescribed by a Clinician to be worn for 6-10 days.
-
Specifically designed to track Parkinsons Disease symptoms like dyskinesia, tremors, motor fluctuations, and more. The data gets sent to your physician so that more accurate information can be collected to inform treatment.
-
The device has the attention of a number of health bodies , and Global Kinetics reports they have taken over 50,000 ‘PKGs’.
The PKG currently monitors motor symptoms, with users needing to report their non-motor symptoms separately.
While it’s in some ways easier to use an Apple Watch for continuous tracking over weeks or months including for related health symptoms, this is a focused, medical-grade device, which may prompt a more medically oriented focus to capture and review symptoms, including advice for Medical professionals to interpret. It may also be of benefit for data capture remotely during related clinical trials.
The information collected by the PKG® Watch can tell your doctor more about your movement during the day including if you are moving a bit slowly or having difficulty in performing movements. The PKG® can help your doctor assess the current state of your Parkinsons disease and assist them in making a decision about the right type and amount of medication for you at the right time.
Important Points About The New Medications
With multiple new medications available for the treatment of PD, there is more hope than ever that Parkinsons symptoms can be successfully managed for many years. A few things to consider:
- For people whose symptoms are difficult to control, these new treatments are welcome additions to what was previously available and many people with PD have been using these new medications with significant benefit.
- On the other hand, many of the newly-approved medications have the same mechanisms of action as older medications so they are not breaking new ground in treating symptoms.
- In addition, for some people, the effect on symptoms may be mild or not substantial.
These caveats may mean that your physician has not suggested a medication change for you. It is also important to note that despite all the new medications, carbidopa/levodopa remains the most potent medication to treat the motor symptoms of PD.
If your doctor does choose to try one of the new options, there may be multiple paths that your doctor can take when contemplating a medication adjustment. Often trial and error is the only way to determine the best medication regimen for you, so you may need to practice some patience as you work together with your doctor to determine what works or doesnt work.
This research also has implications for Alzheimers disease, Type 2 diabetes and other serious human diseases where symptoms are triggered by protein misfolding.
Explore further
Don’t Miss: Is Melatonin Safe For Parkinson’s Patients
Iv Paper Selection And Classification Methodology
This section presents our methodology to select and classify papers that use technology for PD research. Figure 2 shows an overview of the selection and classification process. We start with an automated search of articles from PubMed Central, Science Direct, IEEE Xplore, and MDPI databases. First, articles that are not relevant to our study are eliminated. For example, articles that include non-human subjects are excluded. In the next step, any duplicate articles that are included in the pool of papers are removed. Finally, the remaining articles are manually classified into one of the four application categories introduced in Section II. In addition, we also mark the mobile technology used in each study according to the categories presented in Section III. We describe each of these steps in more detail in the following sections.
Flow diagram of the systematic review process to new technologies used in assessment of Parkinsons Disease in the last ten years
