Levodopa Usually Is Best First Therapy For Motor Symptoms Neurology Group Says
byJudy George, Senior Staff Writer, MedPage Today November 15, 2021
Neurologists should counsel people with early Parkinsons disease about the benefits and risks of starting treatment with levodopa, dopamine agonists, or monoamine oxidase B inhibitors for motor symptoms, new guidelines from the American Academy of Neurology stated.
If treatment is started, levodopa is the preferred initial therapy for most early Parkinsons patients, wrote Tamara Pringsheim, MD, of the University of Calgary in Alberta, Canada, and co-authors in Neurology.
We carefully reviewed the available research on the effectiveness and possible risks of medications to treat motor symptoms in people with early Parkinsons disease and found that levodopa is usually the best first treatment for these symptoms, Pringsheim said in an AAN statement.
Still, there are side effects with levodopa as well as other drugs, so it is important that a person newly diagnosed with Parkinsons disease discusses all options with their neurologist before deciding on the best treatment plan for them, she added.
The guidelines update AAN practice parameters from 2002. Since then, new medications and new formulations of older medications have become available, Pringsheim and colleagues noted.
The analysis showed:
- Levodopa was better at reducing motor symptoms than dopamine agonists, with most studies demonstrating significantly greater improvement in UPDRS part III scores for up to 5 years of follow-up
Our 12 Takeaways From The Aan 2019 Annual Meeting
1. A First in Human Study of PBT434, a Novel Small Molecule Inhibitor of alpha-synuclein aggregation
Accumulation of alpha-synuclein may be the main problem that causes nerve death in PD. PBT434 had been tested in animal models of PD and was shown to decrease alpha synuclein accumulation, preserve neurons and improve motor function. This current trial is the first time the molecule has been given to humans. The trial was done in healthy controls and was found to be well tolerated.
Takeaway: Although a long way from being a medication to help people with PD, it is encouraging to learn about brand new approaches to treating PD that are being developed and tested. The next step will be to test this molecule in people with PD.
2. Once Daily Opicapone Increases ON-Time in Patients with Parkinsons Disease: Results from Two Phase 3 Studies
Opicapone is a newly developed Catechol-O-methyltransferase inhibitor which acts to stop the breakdown of levodopa, allowing it to prolong the effects of a dose. Two phase 3 trials were presented in which opicapone was tested in hundreds of patients to determine if opicapone is safe and effective. Results showed that opicapone increased the amount of time in the day that levodopa worked well, without increasing troublesome dyskinesias, although an increase in dyskinesias generally was reported.
Takeaway: With two phase 3 trials showing positive effects, opicapone is now being evaluated by the FDA for possible approval.
Takeaways:
A Review On Parkinsons Disease Treatment
5214526Tori K. Lee Eva L. Yankee
Department of Biology, Angwin, CA 94508, USA .
Received:First Decision:Revised:Accepted:Available online:Academic Editors:Copy Editor:Production Editor:
© The Author 2021. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Also Check: Can Acupuncture Help Parkinson’s
Classes Of Antiparkinsonian Drugs
Several drugs are used for treatment of PD and classified into dopaminergic and nondopaminergic. The dopaminergic drugs include levodopa, dopaminergic agonists , monoamine oxidase-B enzyme inhibitors, and catechol-ortho-methyltransferase inhibitors. Nondopaminergic drugs are amantadine and anticholinergics.
In Brazil, antiparkinsonian drugs are available on the Public Health System, except for extended release pramipexole, safinamide, and rotigotine.
Pharmacological Treatment Of Parkinsons Disease
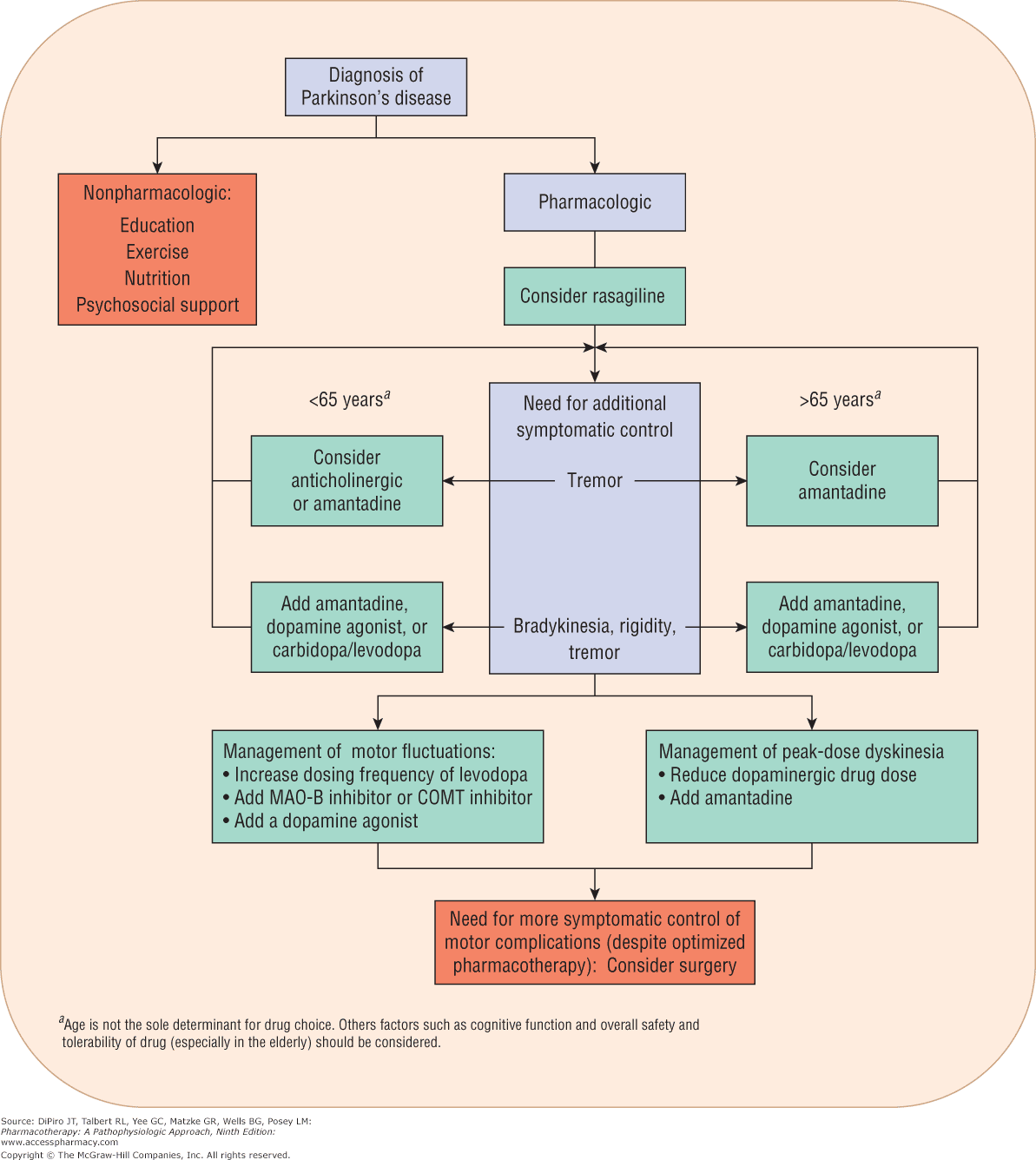
There is currently no proven disease-modifying or neuroprotective therapy for PD. A summary of previous neuroprotection trials is given in a recent review article. Current evidence-based treatment for PD is symptomatic and mainly based around dopaminergic replacement or modulation . The evidence base is summarised in recent guidelines from the National Institute for Health and Care Excellence and the International Parkinson and Movement Disorder Society. Levodopa, dopamine agonists and monoamine oxidase B inhibitors are all licensed for use as initial therapy in PD. Anticholinergics are no longer routinely used due to the risk of cognitive decompensation.
Pharmacological therapies currently used for initial and adjunctive treatment of motor symptoms in Parkinsons disease
Dont Miss: Does Dennis Quaid Have Parkinsons
You May Like: When To Start Parkinson’s Medication
Manual Therapy And Exercise
Chiropractic manipulation, osteopathic manipulation, and Trager therapy have been suggested to benefit patients with Parkinsons disease. No studies exist, however, to refute or confirm this position. The Alexander technique has shown some benefit and patient improvement has been noted in some studies.
Standard physical therapy, as well as occupational therapy, did result in improved functional outcomes, but the benefit was small and was not sustained when the exercise therapy stopped.
Systematic Review: Efficacy And Safety Of Medical Marijuana In Selected Neurologic Disorders
Current systematic review. Endorsed by the American Autonomic Society, the American Epilepsy Society, the Consortium of Multiple Sclerosis Centers, the International Organization of Multiple Sclerosis Nurses, and the International Rett Syndrome Foundation. Reaffirmed January 21, 2014, and January 11, 2020.
Also Check: Parkinson’s Sleeping All The Time
Motor Fluctuations And Dyskinesia
For the treatment of motor features of tremor, bradykinesia, and rigidity associated with Parkinsons disease, dopaminergic therapies are initially effective however, motor fluctuations eventually complicate therapy and can cause significant disability and impair quality of life. Sustained-release carbidopa/levodopa and bromocriptine have not been found to reduce off time.
Risk factors for motor complications include disease severity, younger age at onset of Parkinsons disease, high levodopa dosage, and longer disease duration. The motor fluctuations usually are addressed with levodopa adjustments as well as adjunctive medications or surgery as discussed below.
Deep Brain Stimulation For The Treatment Of Pd Patients
Current surgical indications for PD include reducing motor fluctuations, off time, dyskinesias, tremor, and improvement of levodopa-responsive symptoms. Deep brain stimulation is probably the most critical advance in treatment of PD since the introduction of levodopa. The beneficial effects of DBS on motor symptoms and quality of life in advanced PD have been shown in randomized, controlled studies6666. Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep-brain stimulation for Parkinsons disease. N Engl J Med. 2006 Aug 31 355:896-908. https://doi.org/10.1056/NEJMoa060281 ,6767. Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinsons disease : a randomised, open-label trial. Lancet Neurol. 2010 Jun 1 9:P581-91. https://doi.org/10.1016/S1474-442270093-4 .
Read Also: What Does Parkinson’s Stiffness Feel Like
American Academy Of Neurology Issues Guidelines For Treating Motor Symptoms
The guidance updates the recommendations on dopaminergic medications that were published in 2002 on the initiation treatment for Parkinson disease.
The American Academy of Neurology has issued guidelines for treating movement symptoms, known as motor symptoms, for individuals with early Parkinson disease.
The guidelines update the recommendations on dopaminergic medications that were published in 2002 on the initiation treatment for Parkinson disease.
We carefully reviewed the available research on the effectiveness and possible risks of medications to treat motor symptoms in people with early Parkinson disease and found that levodopa is usually the best first treatment for these symptoms, Tamara Pringsheim, MD, MSc, of the University of Calgary and a fellow of the AAN, said in a statement.
The new guidelines recommend that health care providers should counsel individuals with early Parkinson disease on the benefits and risks of 3 initial therapy options: dopamine agonists, which mimic the effects of dopamine levodopa, a drug that is converted into dopamine in the brain and monoamine oxidase B inhibitors, which prevent MAO-B enzymes from breaking down dopamine.
The guidelines also state that treatment with levodopa provides superior benefits at reducing motor symptoms than the other options.
The guidelines recommend that health care providers prescribe the lowest effective dose to minimize the risk of dyskinesia and optimize benefits.
Patient Population And Demographics
Demographics, clinical characteristics, and CSF biomarker levels of the study discovery and validation CSF cohorts are summarized in . The discovery CSF cohort included 62 patients with PD and 34 controls. The average age of the PD discovery cohort was 57 ± 10 years, with average H& Y stage 2 ± 0.5, average UPDRS-III score 23.5 ± 9.1, and average Montreal cognitive assessment score of 25.8 ± 2.9 with 36 male and 26 female participants. Controls were aged 50 ± 16 years, with 16 male and 18 female participants. The validation CSF cohort included 49 patients with PD with an average age of 63 ± 10 years, average H& Y stage 2 ± 0.5, average UPDRS-III3 score of 21.7 ± 8.6, and average MMSE score of 28 ± 1.6 with 30 male and 19 female participants. HCs were aged 50 ± 16 years, with 16 male and 32 female participants.
Scatterplots showing the correlation analysis in the discovery cohort between CSF-seeded Syn oligomers and UPDRS motor scores and H& Y scores, respectively . Scatterplots showing the correlation analysis in the validation cohort between UPDRS motor and H& Y scores with seeded CSF Syn oligomers . The subplots present the same dataset excluding extreme data points highlighted with the red square. The solid line highlights the calculated regression line. p Values and Spearman rs are displayed for each correlation. H& Y = Hoehn and Yahr UPDRS-III = Unified Parkinson Disease Rating Scale Part-III.
Read Also: Pemf Therapy For Parkinson’s Disease
Syn Seed Amplification Assay
eTable 1 summarizes the differences between the different seed amplification protocols used in this study. For BH samples, we followed a modified version of Shahnawaz et al., a well-established protocol for Syn seeding amplification assay. In brief, 160 L of reaction mix composed of 0.1 M piperazine-N, N bis , pH 6.5, 0.5 M sodium chloride , 10 M thioflavin T , and 0.1 mg/mL wild-type untagged monomeric Syn were distributed in a 96-well black plate with clear bottom at a final volume of 200 L per well. For each test, we loaded 40 L of BH of 0.1 mg/mL total protein concentration. The plate was then sealed with a sealing tape and incubated in Omega FLUOstar plate reader at 37°C for 120 hours with intermittent shaking cycles: double orbital with a 1-minute shake and 15 minutes rest throughout the indicated incubation time.
Without Guidelines Docs Make Their Own Long Covid Protocols
Their work is urgent. In the U.S. alone, as many as 29 million people have long COVID, according to estimates from the American Academy of Physical Medicine and Rehabilitation.
âPatients with long COVID have on average at least 14 different symptoms involving nine or more different organ systems, so a holistic approach to treatment is essential,â says , MD, executive director of the Post-COVID Rehabilitation and Recovery Clinic at the University of Washington in Seattle.
For acute COVID cases, the National Institutes of Health has treatment guidelines that are taking a lot of the guesswork out of managing patientsâ complex mix of symptoms. This has made it easier for primary care providers to manage people with milder cases and for specialists to come up with effective treatment plans for those with severe illness. But no such guidelines exist for long COVID, and this is making it harder for many doctors â particularly in primary care â to determine the best treatment.
While there isnât a single treatment that is effective for all long COVID symptoms â and nothing is approved by the FDA specifically for this syndrome â doctors do have tools, Friedly says.
Fatigue is an obvious target. Widely available screening tools, including assessments that have been used in cancer patients and people with chronic fatigue syndrome, can pinpoint how bad symptoms are in long COVID patients.
Show Sources
Recommended Reading: How To Avoid Getting Parkinson’s
Tapering And Discontinuing Das
Recommendation 5 Rationale
Adverse effects associated with DAs can lead to substantial impairments in psychosocial functioning, interpersonal relationships, and quality of life for the patient and caregivers. The consequences of medication-related adverse effects may be mitigated through adjustments to prescribed medications, including DAs, or through additional behavioral or pharmacologic interventions, if appropriate.
Patients may experience undesirable side effects when attempting to decrease dopaminergic medications, especially DAs, including dopamine agonist withdrawal syndrome or low mood and apathy. These side effects can make it difficult to taper or discontinue DAs. Staged reduction in dosing may reduce the severity of withdrawal symptoms and improve compliance with medication recommendations.
Recommendation 5 Statements
-
5a. Clinicians should recommend tapering or discontinuation of DAs if patients experience disabling medication-related adverse effects, including ICDs, EDS, sudden-onset sleep, cognitive impairment, or hallucinations .
-
5b. When DAs must be discontinued due to adverse effects, clinicians should monitor patients for symptoms of DAWS and, when possible, gradually decrease the dosage to minimize symptoms .
Aan Publishes New Guideline For Treatment Of Early Parkinson Motor Symptoms
The guideline updates previous recommendations for dopaminergic medications published in 2002, integrating new medications and formulations.
The American Academy of Neurology has issued a new clinical practice guideline with recommendations for treatment of motor symptoms in early Parkinson disease this week. This new guidance updates an existing practice guideline published in 2002 that outlines initiation of treatment for early PD and use of dopaminergic medications.1,2
The panel of authors assigned 3 levels of obligation for recommendationsA, B, and Cusing the Delphi process. Level A represents the strongest recommendations, which use the word must due to high confidence in evidence that suggest high benefit and low risk for patients. There were no Level A recommendations made within the guideline. Level B recommendations encompass what clinicians should do while they are not as strong as Level A recommendations, they are still based on a similar benefit-risk profile . Level C represents the lowest recommendations, with practices clinicians may want to consider.2
REFERENCE
Also Check: Psilocybin And Parkinson’s Disease
Aan Issues New Guideline For Treatment Of Early Pd
We were unable to process your request. Please try again later. If you continue to have this issue please contact .
The American Academy of Neurology has issued a guideline providing recommendations for treating motor symptoms in people with early Parkinsons disease.
The guideline, published in Neurology, was endorsed by the Parkinsons Foundation. It updates recommendations on dopaminergic medications that were first published in a 2002 AAN guideline on the initiation of treatment for PD.
Dopamine-releasing medications have been used to relieve initial PD symptoms, such as motor symptoms including tremor, rigidity and bradykinesia.
We carefully reviewed the available research on the effectiveness and possible risks of medications to treat motor symptoms in people with early and found that levodopa is usually the best first treatment for these symptoms, guideline lead author Tamara Pringsheim, MD, MSc, of the University of Calgary and a fellow of AAN, said in a press release. Still, there are side effects with levodopa as well as other drugs, so it is important that a person newly diagnosed with discusses all options with their neurologist before deciding on the best treatment plan for them.
According to the new recommendations, treatment with levodopa was found to provide superior benefits in reducing motor symptoms when compared with treatment with either dopamine agonists or MAO-B inhibitors.
Therapeutic And Formalized Pattern Exercises
The SPARX study enrolled 128 de novo patients and compared high- and moderate-intensity treadmill exercises with a wait-list control group. After six month of 3 days per week exercise, the results showed that the high-intensity group, who exercised at 80 to 85% maximum heart rate, had less change in motor symptoms compared with the usual care group9898. Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 2018 Feb 1 75:219-26. https://doi.org/10.1001/jamaneurol.2017.3517 . The Park-in-shape trial , a home-based study, recruited 130 PD patients in Hoehn & Yahr stage 2 who were randomized either to exercise on a stationary cycle or stretching at least three times per week. After the 6-month program, the MDS-UPDRS motor score change was smaller in the aerobic group, resulting in a between-group adjusted mean difference of 4.2 points favoring the cycling group9999. Van der Kolk NM, de Vries NM, Kessels RPC, Joosten H, Zwinderman AH, Post B, et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinsons disease: a double-blind, randomised controlled trial. Lancet Neurol. 2019 Nov 1 18:P998-1008. https://doi.org/10.1016/S1474-442230285-6 .
You May Like: What Part Of The Brain Does Parkinsons Disease Affect
You May Like: Pacemaker For Parkinson’s Disease
Description Of The Analytic Process
In August 2017, the AAN Guideline Subcommittee recruited a multidisciplinary panel of authors to develop this guideline. The panel included content and methodology experts, patient representatives, and a staff representative from the Michael J. Fox Foundation for Parkinsons Research. As required by the AAN, a majority of the members of the panel and the lead author are free of conflicts of interest relevant to this practice guideline. Five of the guideline developers were determined to have COI, but the COI were judged to be not significant enough to preclude them from authorship . Whereas the development of this guideline primarily followed the 2017 edition of the AANs Clinical Practice Guideline Process Manual, this edition of the manual was not published by the time of the guideline initiation. Therefore, disclosures were reviewed following the previous process found in the 2011 Clinical Practice Guideline Process Manual. The full author panel was solely responsible for the final decisions about the design, analysis, and reporting of the systematic review and practice guideline, which was submitted for approval to the AAN GS.
