What Is Parkinsons Stem Cell Treatment
The symptoms of Parkinsons disease result from the damage and death of specialized nerve cells in a part of the brain called the substantia nigra pars compacta. When these nerve cells cease to function properly, certain messages from the brain are no longer effectively transmitted to the body. This results in a number of distinctive symptoms including tremors, balancing difficulties, and loss of general mobility. Stem cells may have the potential to repair some of the CNS damage brought on by this disease through anti-inflammation, angiogenesis and neurotrophic factors.
Studies Show Promising Results
“Considering the ability of MSCs to secrete neurotrophic factors, modulate inflammation, and possibly even act as mitochondria âdonorâ, it comes as no surprise that there is a lot of interest in the use of MSCs in the treatment of Parkinsons Disease, and a multitude of animal studies has shown promise. Treatments have resulted in improvement of motor function, protection of the nigrostriatal system, and improved striatal dopamine release in several studies using toxic lesion rodent models of Parkinsons Disease. Similar effects were reported with umbilical cord-derived MSCs with or without prior differentiation. For example, a recent study reported improvement of motor function, reduced microglial activation, and decreased loss of TH immunoreactivity, associated with local production of trophic factors.
Learn more about DVC Stem’s protocol for Parkinson’s Disease here:
References:
Venkataramana, N. K., Kumar, S. K. V., Balaraju, S., Radhakrishnan, R. C., Bansal, A., Dixit, A., ⦠Totey, S. M. . Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Retrieved from https://www.sciencedirect.com/science/article/pii/S1931524409002205#!
Unified Parkinson’s Disease Rating Scale. . Retrieved from https://www.sciencedirect.com/topics/medicine-and-dentistry/unified-parkinsons-disease-rating-scale
About the author
Moral Positions Concerning The Use Of Hesc For Medical Treatment
Participants moral positions regarding the use of embryos for therapy development were diverse. It was perceived as a complex and difficult issue that some individuals had never thought of, and it triggered thoughts and feelings. One person asked herself how much one should change the course of nature.
How far should we go in changing , as in genetics, uhm, where do we draw the line on what is ethical, that is what I think about a lot…
Some had no firm opinions, while others were clear about their positions. More knowledge of the effects of the treatment was important for some to make a decision. Some thought it should be up to the experts, researchers or legislators to decide whether the embryos should be used for medical purposes.
It is up to the researchers to use them in a right way…one has to follow the current legislation. I have no concerns in my conscience regarding this.
So my first was that I will not say it was all negative, but it was in the negative direction this feels a bit tricky. What are they going to do with it? What are they after? I dont understand.
In this case, a person with Parkinsons has lost something existing if you can replace it in this way, then I dont see an ethical problem with it at all
I find it harder to accept that you, in some way, improve nature, but replacing what has been lost is not problematic at all to me.
Recommended Reading: Can Concussions Cause Parkinson’s Disease
Parkinsons Disease: How Could Stem Cells Help
What do we know?
Tremors, muscle rigidity and other symptoms of Parkinsons disease are caused by the death of dopamine-producing neurons in the brain. Dopamine producing neurons throughout the brain are affected, but the substantia nigra is the primary brain region where neurons are lost.
People affected by PD often develop abnormal protein clumps in their brain called Lewy bodies. These clumps are made of a protein called alpha-synuclein.
Levodopa is the primary drug used to treat PD. Levodopa is converted into dopamine when in the body, which compensates for lost dopamine-producing neurons.
What are researchers investigating?
Approximately 5% of people with PD have inheritable gene mutations linked to PD. Researchers are investigating what causes PD in the other 95% of patients in clinical studies, animal models and cell models.
Transplantation of young brain cells from human foetuses into people with PD has shown promising results in previous clinical trials. The current TRANSEURO study is re-examining this treatment method with the aim of minimising side effects and measuring efficacy.
Scientists can now make dopamine-producing neurons from both human embryonic stem cells and human induced pluripotent stem cells . Neurons made from human ESCs and iPSCs mature into human dopamine-producing neurons, survive and function after transplantation into mouse, rat and monkey models of PD.
What are the challenges?
Replacing lost cells
Is It Safe Safety Of Commercial Stem Cell Clinic Work
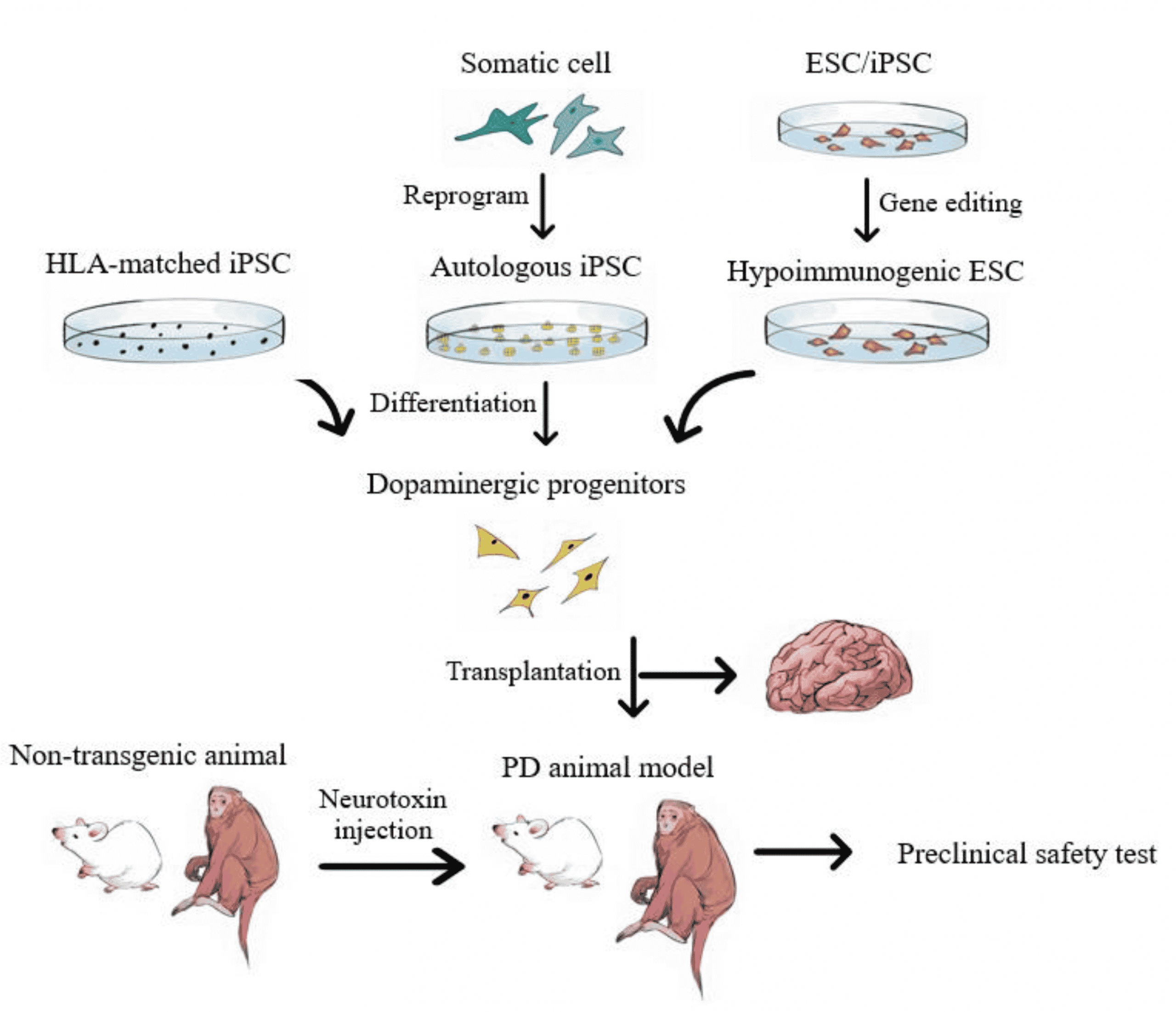
Safety data is also limited, although there have been some publicized lawsuits claiming that these treatments resulted in harm. Stem cell researchers in general question whether cells harvested in such a way contain sufficient amounts of adult-derived stem cells to be meaningful. It is also unclear how this type of procedure would target the stem cells to the correct location. If stem cells are introduced in the nose for example, it is unclear how they would find their way to the basal ganglia and make the correct connection in order to help a person with Parkinsons disease.
In order for the medical community to accept this type of treatment as safe and beneficial, it would need to be shown to work in a placebo-controlled clinical trial for which participants do not pay, are aware of the known risks and benefits, and are carefully monitored throughout the trial. In addition, the trial would need to track adverse events, as well as record and share the outcomes of trial participants as they compare to the group of patients receiving a placebo treatment. So far this has not happened. The FDA is in fact studying mesenchymal stem cells in the laboratory in order to determine the best way to use them to help people, but these studies have not yet led to approved treatments. Most recently, the FDA filed federal complaints against two clinics that are marketing stem cell products without regulatory approval.
Recommended Reading: Does Parkinson’s Affect Breathing
What Have Clinical Trials Found
Until the discovery of the process of creating iPSCs, the only stem cell therapies for Parkinsons disease required the use of embryonic stem cells. This came with ethical and practical challenges, making research more difficult.
After iPSCs became available, stem cells have been used in clinical trials for many conditions involving neural damage with overall mixed results.
The first clinical trial using iPSCs to treat Parkinsons disease was in 2018 in Japan. It was a very small trial with only seven participants. Other trials have been completed using animal models.
So far, trials have shown improvement to symptoms affecting movement as well as nonmotor symptoms such as .
Some challenges do arise from the source of the stem cells.
Stem cell therapy can be thought of as being similar to an organ transplant. If the iPSCs are derived from a donor, you may need to use immunosuppressant drugs to prevent your body from rejecting the cells.
If the iPSCs are derived from your own cells, your body might be less likely to reject them. But experts believe that this will delay stem cell therapy while the iPSCs are made in a lab. This will probably be more costly than using an established line of tested iPSCs from a donor.
Stem Cell Research And Parkinsons
The aim of stem cell research in Parkinsons is to understand how nerve cells develop, why some die and how healthy cells can be used to replace damaged brain cells. With this knowledge it may be possible to replace the damaged cells in the brain by introducing healthy dopamine-producing cells generated from stem cells grown in the laboratory. Healthy dopamine-producing cells derived from stem cells could also be useful to researchers in testing new treatments.
Researchers are particularly interested in embryonic stem cells as they have the potential to develop into all types of cells in the body, including the brain. More research is needed in order to understand the way these cells work to ensure that replication can be controlled and a safe treatment developed.
Dont Miss: Diseases Similar To Parkinsons
Read Also: What Are The Beginning Signs Of Parkinson’s Disease
New York State Stem Cell Science Consortia
Members of the New York State Stem Cell Science team at Memorial Sloan Kettering — Isabelle Riviere, Stefan Irion, Mark Tomishima, Laurel DeGeorge, Abderrahman El-Maarouf and Michel Sadelain Viviane Tabar, Urs Rutishauser, Claire Henchcliffe, Lorenz Studer
Parkinsons disease is the second most common neurodegenerative disorder and is estimated to affect more than four million patients worldwide a number predicted to more than double by 2030. A fundamental characteristic of PD is progressive, severe, and irreversible loss of specific dopamine-producing neurons in the midbrain that ultimately may result in disabling motor dysfunction. Multiple therapies have been developed for PD, but none can replace the lost cells. Cell transplantation has been considered a promising therapy, but in spite of extensive efforts to develop it in laboratories across the world, this approach has faced multiple challenges, including the absence of an appropriate cell source that can match the lost cells in function and safety.
In 2011, our team made a major discovery that enables the derivation of nearly unlimited numbers of authentic, engraftable midbrain DA neurons from human embryonic stem cells . In recent publications, we have demonstrated that these cells can survive in three independent PD models and can reverse motor deficits of the disease.
Stem Cell Treatments In The Future Of Parkinsons Disease Management
Although there are a number of challenges brought about with stem cell-based treatments for PD, it seems probable that these treatments will progress to the clinic in the short- to medium-term future. While development of optimized products has been necessarily slow and iterative, the field is now asking questions about how these treatments can be scaled and deliveredthis demonstrates the progress that has been made with these approaches.
As has been discussed, the purpose of stem cell treatments is predominantly to treat the motor symptoms of PD. They will not have any disease-modifying effect and will not treat the major non-motor symptoms which can be particularly disabling in some patients. While these techniques can form one arm of the future of PD treatment, they will likely be combined with other novel treatments targeting alpha-synuclein pathology . It may be possible for stem cell-based regenerative therapies to be employed to restore the function of dopaminergic neurons that have already been lost, while novel disease-modifying drugs could be used to prevent ongoing neuronal death.
Read Also: What Is The Difference Between Essential Tremors And Parkinson’s
Interests Related To The Use Of Hesc For Medical Treatment
The participants identified several interests related to the use of human embryos for development of medical therapies. They balanced the interests they identified against each other during the interviews. Their interests concerned not only them as patients but also brought up interests of others.
Interests relating to themselves as patients
The participants expressed a need for improved medical treatments against PD. Some described that the medicines they had tested so far had no, or insufficient, effect. The efficacy of the treatment was described as important, and there were concerns that these treatments would not be efficacious enough.
Patient safety was important, and participants worried about potential side-effects of the treatment. Injecting substances into the brain was perceived as something risky. Some were solely worried about having to undergo a brain surgery others were concerned about the substance and its short- and long-term side-effects.
what happens in the body what happens in the long run in the next generation, is it something you carry with you everyone who has Parkinsons is not 78, but there are some that get Parkinsons very early on.
If they then have children, what does this treatment do to the next generation.
Participants also worried about cell rejection, becoming ill, the cells being put in the wrong place or losing their functions, such as limiting the ability to go biking or swimming.
Patients interests related to the donors
The Next Generation Of Trials
Studer was part of the initial studies involving fetal tissue in the 1980s and 1990s, and knew from the start that the work was more of a proof of principle than a solution for people with Parkinsons. For me it was clear that a fetal transplant isnt a long-term solution because of ethical, legal and practical issues. Because this procedure requires 4 to 12 fetuses per patient, there was no way they could treat thousands, let alone tens of thousands, of people that way. Instead, Studer turned to stem cells.
Immunosuppression is a particularly important element of BlueRocks approach, because it relies on a single cell line that cannot be adjusted to more closely resemble the recipients own tissues. A group led by stem-cell scientist and neurosurgeon Jun Takahashi at Kyoto University in Japan is attempting to provoke a lesser immune response by pairing transplant recipients with cells that are less likely to be rejected. The researchers are using cell-surface proteins, called major histocompatibility complexes , that are recognized by the adaptive immune system and can have varying levels of compatibility from one person to another. Rather than using frozen cell lines, Takahashi and his colleagues are creating a fresh batch of MHC-matched cells for each transplant.
Read Also: How Many Stages Of Parkinson’s Is There
What Causes Parkinsons Disease
Parkinsons Disease is caused by a loss of nerve cells in the brain. This loss of nerve cells within the brain results in a reduced amount of dopamine being created which acts as a messenger between the parts of your brain that control voluntary and involuntary movement. Therefore without that vital connection, your brain starts losing the ability to effectively control movement. Currently, it is unknown what causes the deterioration of nerve cells associated with Parkinsons Disease . Currently, it is believed that both environmental factors, as well as genetic factors, may play a role in the loss of nerve cells.
Parkinsons Disease is a lifelong condition that can greatly impair the ability of ones daily functions. Traditional treatments only address the symptoms of the condition, but researchers are excited about the possibilities of certain gene therapies and stem cell therapy, which may have the ability to reverse damage and halt the progression of the disease.
Ipscs As Disease Model For Parkinsons Disease
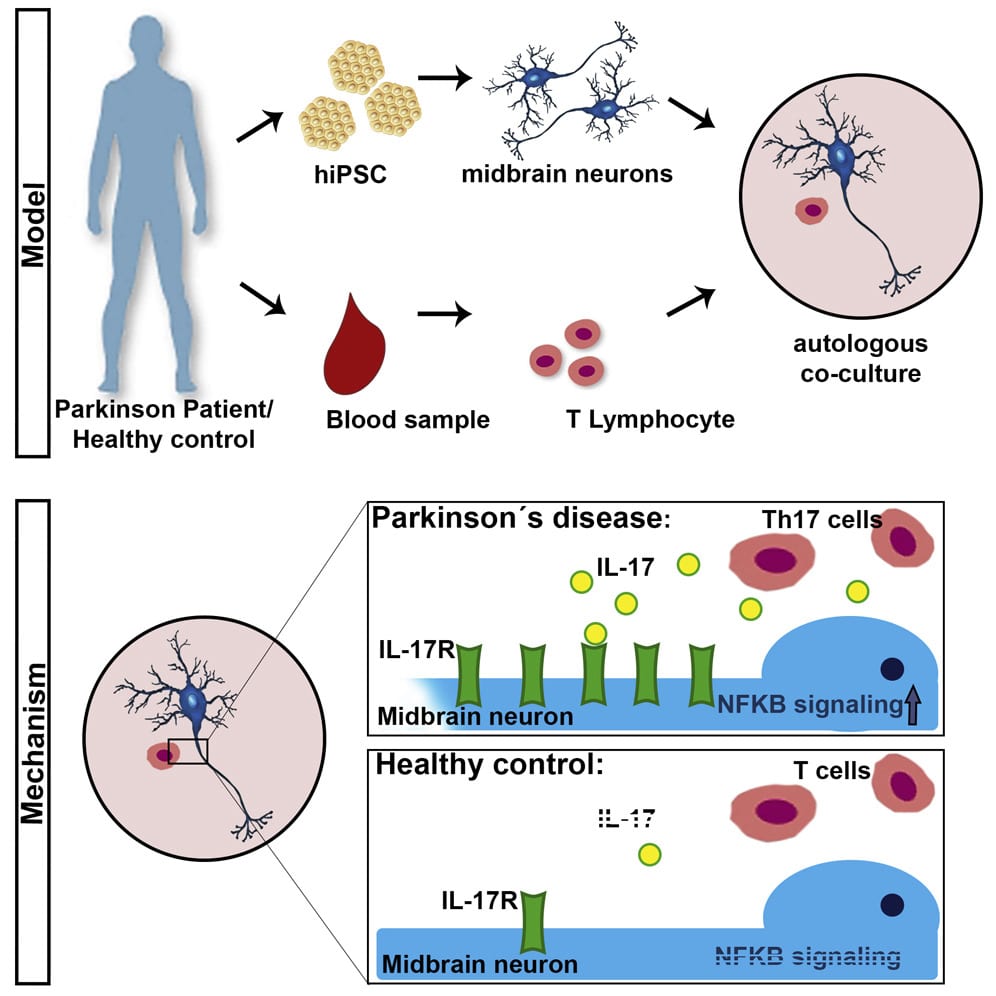
The contribution of pharmacogenomics has been heightened through the use of patient-specific iPSC lines and genetic engineering technology to manipulate them. Ever since Yamanakas discovery in 2007 that a handful of transcription factors can reprogram cellular differentiation, iPSCs have been utilized extensively in the study of neurodegenerative disease to direct patient-specific cell fate . While still limited in scope, iPSCs are currently the most robust and phenotypically similar model for PD . Mutations of consequence can now be captured in iPSC lines and directed by small molecules to a DAn fate in PD modelsall within a dish. Displayed openly, the real-time cellular effects of mutation can be physically observed and studied in tandem with control lines to limit genetic background effects of the affected individual similarly, effects of oxidative stress common to PD can also be quantified with broad clinical applications for drug screening without human side-effects. Not surprisingly, it remains difficult to physically confirm the mechanisms of neurodegeneration and neuroprotection implicated by iPSC research as patients neurons are hidden deep within the brain. These effects similarly cannot be perfectly translated into the cellular environment of PD due to some epigenetic effects of aging eliminated in reprogramming protocols.
You May Like: Does Parkinsons Cause Swelling
Recommended Reading: How Fast Does Parkinson’s Develop
What Causes Parkinson’s Disease
Parkinson’s Disease is caused by a loss of nerve cells in the brain. This loss of nerve cells within the brain results in a reduced amount of dopamine being created which acts as a messenger between the parts of your brain that control voluntary and involuntary movement. Therefore without that vital connection, your brain starts losing the ability to effectively control movement. Currently, it is unknown what causes the deterioration of nerve cells associated with Parkinson’s Disease . Currently, it is believed that both environmental factors, as well as genetic factors, may play a role in the loss of nerve cells.
Parkinson’s Disease is a lifelong condition that can greatly impair the ability of one’s daily functions. Traditional treatments only address the symptoms of the condition, but researchers are excited about the possibilities of certain gene therapies and stem cell therapy, which may have the ability to reverse damage and halt the progression of the disease.
Cell Assessment Of Differentiated Da Neurons
Understanding the key type of DA neurons required to achieve downstream restoration of PD pathology is essential. The mesotelencephalic DA system in the midbrain contains two main groups: the A9 neuronal clusters of the nigrostriatal DA pathway located in the zona compacta, the substantia nigra involved in the control of posture, and the A10 neurons located in the ventromedial mesencephalic tegmentum that regulates the locomotor activity and emotional behavior . Dysfunction of the nigrostriatal system has been linked to Parkinsonism and later to schizophrenia, drug addiction, and depression . Differences between the two DA cell populations have been observed in neurochemistry and in spontaneous neuronal firing . More importantly, A9 neurons display significantly enhanced levels of neuromelanin pigmentation as compared to other dopamine-producing neurons . This could account for the association of early loss of A9 DA neurons in Parkinsons disease with increased vulnerability upon disease progression with the relative preservation of A10 DA neurons .
Generally, stem cells are differentiated into specific nigra A9 DA neurons in large quantities prior to PD transplantation. This step has been thoroughly reviewed by many articles such as in Fan et al. and, thus, will not be further discussed here. However, we focus on developments in technology in cell assessment of differentiated DA neurons.
You May Like: Judy Woodruff Parkinsons
You May Like: Parkinson’s Disease Treatment Options
