When To See A Doctor About Parkinsons
There isnt one specific test to diagnose Parkinsons disease. Doctors will usually evaluate your symptoms and perform several tests to determine if you have the condition. If you notice the following early warning signs, then you should see a doctor.
The early warning signs of Parkinsons disease include:
Genetic Classification Of Pd
In the current PD genetics nomenclature, 18 specific chromosomal regions, also called chromosomal locus, are termed PARK , and numbered in chronological order of their identification . In addition to being an incomplete list of known PD-related genes, this classification system, unfortunately, has a number of inconsistencies. It comprises confirmed loci, as well as those for which linkage or association could not be replicated . The causative gene has not yet been identified for all of the loci, nor do all of the identified genes contain causative or disease-determining mutations . Finally, one locus, PARK4, was designated as a novel chromosomal region associated with PD but was later found to be identical with PARK1 . It is noteworthy that some of the loci have been identified by genetic linkage analysis in large families, some based on the known function of the protein product of the gene they contain, yet others have been established by genome-wide association studies performed on a population level. A list of the PARK PD-related genes and loci is given in , along with their clinical classification, inheritance pattern , gene , status , and mode of identification.
Genetic Role Not Entirely Known In Affected Families
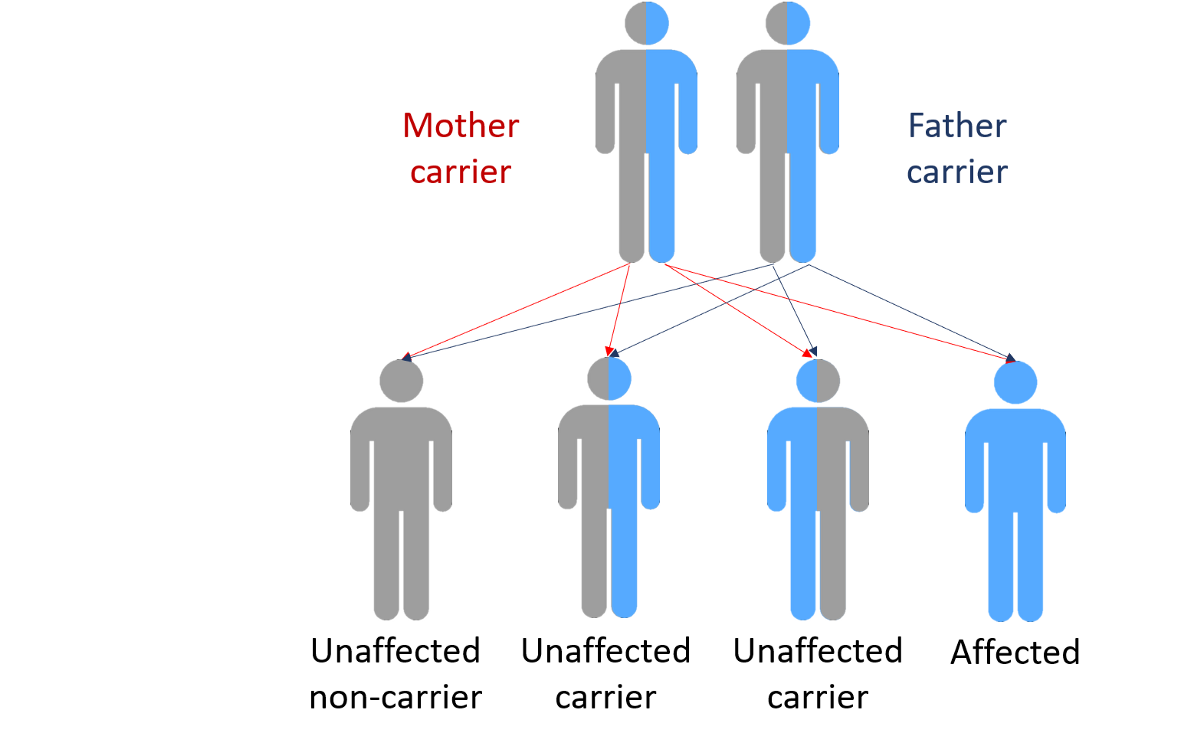
Genetics very likely plays a role in all types of Parkinson’s disease. However, while having a specific combination of genetics may increase your risk of the disease, it doesn’t necessarily mean that you’ll get it.
Around 15 to 25 percent of people living with Parkinson’s have a family history of the condition, either an immediate or second-degree relation. Having one or more of these relatives will place you at slightly higher risk for Parkinson’s, but it’s still no guarantee you’ll develop the disorder.
Conversely, if you have Parkinson’s, it shouldn’t suggest that any of your kids or grandkids will get the disease either. It merely indicates that their risk is slightly above those without a family history.
In the end, most cases of Parkinson’s don’t have any known cause . While there are forms that seem to run in families, these account for a small percentage of cases roughly five to 10 percent, all told.
Recommended Reading: Sinemet Side Effects Elderly
Will Findings From Pd Ipsc Models Translate To Human Clinical Trials
One of the most exciting applications of patient-derived iPSC models of PD is to validate pharmacological treatments before clinical trials. The field is still at the stage of improving human brain tissue engineering, and many different protocols are being tested and developed. However, the need for progress in clinical translation for brain disorders is extremely high, and there is no time to wait for brain tissue models to be perfect. Pioneering iPSC studies pave the road to success and identify limitations which help the community to reach a consensus on the minimal requirements to model brain disorders in vitro most accurately. It seems essential to improve the efficiency of reprogramming and differentiation protocols while trying to make those models as physiological and realistic as possible,. Some concerns are raised that in vitro neuronal development, maturation and function might be too artificial, suggesting that the model may overlook some of the critical processes that occur in vivo. Nevertheless, some defects observed in iPSC-derived neurons have already been confirmed in human postmortem brain tissues,,,,. Although this is very encouraging, it is unclear whether significant in vitro phenotypes that cannot be confirmed in postmortem brain tissue should be disregarded. Most postmortem brains also have technical limitations and may represent later stages of the disease, whereas iPSC models may represent earlier stages, preceding neurodegeneration.
Comprehensive Research Synopsis And Systematic Meta
1Neuropsychiatric Genetics Group, Department of Vertebrate Genomics, Max Planck Institute for Molecular Genetics, Berlin, Germany
2Department of Neurology, Massachusetts General Hospital, Charlestown, Massachusetts, United States of America
3Department of Neurology, Medical Center of the Johannes Gutenberg-University, Mainz, Germany
4Department of Neurology, University Hospital, Münster, Germany
Recommended Reading: Does Parkinson’s Cause Memory Issues
The Interplay Between Genomic Predispositions And Environmental Factors Leads To Parkinsons
In the mid-1990s, the connection between PD and underlying genetic mutations was established,,. It is now evident that varying degrees of the interplay between genomic predispositions and aging and cellular stressors impose a risk for disease . Previous studies have shown vascular insults to the brain, repeated head trauma, neuroleptic drugs, exposure to pesticides, and manganese toxicity increase the risks of developing symptoms of PD,,. In addition, advancing age can also cause a cascade of stressors within the substantia nigra, which weakens the neurons and their ability to respond to further insults,. Ultimately, the uniqueness of the interactions between genes and the environment makes the development of a single treatment for PD difficult as they give rise to a spectrum of neuronal phenotypes that can be unique to individual patients . The development of a model with the ability to replicate the genomic and epigenetic aspects of the disease is crucial . As increasing evidence suggests that genetic mutations are key modulators of disease initiation and progression, the identification and understanding of the various genomic predispositions are required for the development of better-targeted treatments to slow the disease progression.
Fig. 1: A combinatorial spectrum of genetic risks, cellular stressors, and brain cell dysfunctions causes Parkinsons disease.
When Should A Person Seek Genetic Testing
Genetic testing is available for some genes related to Parkinsons disease, but testing may not provide useful information to individuals.
For one thing, a wide range of genes may play a role, and it is not possible to test them all. A person may also have a relevant feature but not go on to develop Parkinsons disease.
For example, only around 0.7% of people with symptoms of Parkinsons disease have changes in the LRRK2 gene, and around 0.3% have changes in the PRKN gene, according to a 2020 review.
Finding out in advance if a young person has the gene may help them prepare for the future if there is strong evidence of a family history of the condition. However, the results are unlikely to be conclusive and may cause unnecessary anxiety.
Anyone who is interested in genetic testing should discuss the pros and cons with a doctor and consider genetic counseling if they decide to go ahead.
The Parkinsons Foundation notes that testing is often hard to access. It can also be costly, and health insurance may not cover it. Genetic counseling can be an additional cost.
Recommended Reading: What Does End Stage Parkinson’s Look Like
People Who Already Have Pd: Should I Get Tested And What Do I Do With The Results
Up until recently, even people with PD with a very extensive family history of PD would not necessarily receive genetic testing because there were no clear uses for the results. There has been research directed at figuring out whether PD caused by or associated with certain mutations have particular clinical characteristics . However, there remains so much variability in clinical characteristics even among people with the same PD mutation, that there are still no clear practical implications in knowing whether a PD patient harbors a particular mutation. There is also, so far, no difference in treatment or management of PD whether or not the patient harbors one of the known mutations. That may change however, with the advent of clinical trials that target particular mutations.
There are two genes that have received particular attention recently because medications are being developed that target those with mutations of these genes.
GBAis a gene that increases the risk of developing PD. The gene encodes for the GBA enzyme, a protein used by the body to break down cellular products. Having two abnormal GBA genes causes Gauchers disease, which is characterized by the buildup of these cellular products resulting in fatigue, bone pain, easy bleeding and an enlarged spleen and liver. When a person inherits only one abnormal gene, he or she does not develop Gauchers disease, but does incur a small increased risk of PD. Most people with one mutated GBA gene do not develop PD.
Genetic Testing May Lead To A Cure
Although genetic testing can leave individuals with many unanswered questions, the data provided may further the study of the disease.
The more individuals you can work, the more things you can discover, says Cannon. We are interested in studying people who have a risk gene because the sooner we can learn how to stop it , the better off people will be.
Clinical trials are in progress to test therapies that target gene mutations, in particular GBA and LRRK2. Pharmaceutical companies conducting these studies need patients who test positive for specific gene variations. By getting tested, individuals have a chance to participate in research programs that may lead to a cure.
Gilbert points out that drugs that target specific mutations may benefit a larger group of Parkinsons patients.
The biochemical problem that happens when a person has an LRRK2 mutation might appear in someone else without an LRRK2 mutation but by another means, she says. So they may also benefit from medication developed for people with an LRRK2 mutation.
If you are interested in participating in a trial, the Michael J. Fox Foundation offers a roundup of the latest investigations currently being conducted and how to get involved.
You May Like: Pontine Syndromes
How Environmental Factors And Aging Can Be Recapitulated In Vitro
An obvious limitation of in vitro models is the lack of environmental context. The influence of nongenetic factors is not recapitulated in the basal phenotype of patient-derived neurons. For example, the influence of head trauma of a boxer with sporadic PD will not be recapitulated by default in reprogrammed neurons. An alternative would be to transplant the patient-derived neurons in animals and simulate the trauma on the animal. Similarly, influence of decades of aging of the human brain is difficult to reproduce in vitro in a few months within the boundaries of feasible experimental design. Brains in a dish will always be an imperfect experimental model. However, many tricks can be used to recapitulate the environmental and aging stress in vitro. Table summarizes a list of reagents that have already been used in iPSC neuronal culture to mimic oxidative stress, proteostatic stress, mitochondrial stress, synaptic stress, ER stress, inflammation, and cellular aging. An interesting example is progerin, a truncated form of lamin A associated with premature aging. Increasing the expression of progerin in iPSC neurons can recapitulate at least some aspect of cellular aging in vitro. Human iPSC-derived dopamine neurons overexpressing progerin displayed specific phenotypes such as neuromelanin accumulation. In addition, PD patient-derived neurons revealed disease-related phenotypes that required both genetic susceptibility and induced-aging in vitro.
Genetic Epidemiology Of Pd
More recently, a number of epidemiological studies with differing methodological approaches and study populations have been published and found to support a familial contribution to PD. In case control studies, positive family history was found to be the single greatest risk factor for PD . In family studies, a family history positive for PD was found in 1024% of patients, and the relative risk for PD in first degree relatives of PD patients ranged from 4 to 10. In the largest of such studies, the frequency of PD was 2% in 1458 first degree relatives of 233 PD patients, a significantly higher frequency than the 1% seen in the 7834 first degree relatives of 1172 age-matched controls .
Sequence of exon 4 of the -synuclein gene from a control individual. Sequence of exon 4 of the mutant allele from an individual affected with Parkinson’s disease from the Contursi kindred. Arrows indicate a transition mutation AG resulting in a missense mutation AlaThr at position 53 of the protein.
Read Also: How Do You Die From Parkinson’s Disease
What To Do If Someone In Your Family Has Parkinsons Disease
If you spot the signs of Parkinsons disease in a member of your family, its important to consult a doctor straight away. Your doctor can formally diagnose the condition and identify where your loved one is on the Parkinsons scale. He or she will also suggest ongoing treatment such as medication and occupational therapy to help ease their symptoms.
The Michael J Fox Foundation Center for Parkinsons Research recommends these tips for caregivers:
- Find a support group for caregivers.
- Stay informed of developments by keeping in close contact with doctors and care staff.
- Stay organized when arranging appointments and physical therapy sessions.
- Expand your medical team to include as many medical professionals as possible.
Therapeutic Implications Of Monogenic Loci
Parkinsons disease is insidious, age-associated and chronic, and consequently a multitude of factors must come into play not only genetic. Nevertheless, the latter provide an unequivocal foundation to elucidate the molecular and cellular biology going awry. So much neuroscience is based on model systems rather than the human condition. Our continued ignorance clearly contributes to past failures in clinical trials and our inability to remedy the condition. Genetic discoveries through linkage, reveal profound insights into the mechanisms of cellular dysfunction and death in parkinsonism, and conversely in the mechanisms required for healthy aging of the basal ganglia. Although these findings are not immediately obvious or intrinsically connected within a pathway or temporal sequence of cellular events, the hope of many is that investigating the molecular consequences of these variants will provide clues as to the commonalities and/or differences in the etiology of these forms of disease. Leading on from this, it is clearly hoped that such an understanding will be directly relevant to the more common, apparently sporadic forms of disease.
Don’t Miss: How Quickly Does Parkinson’s Dementia Progress
Genetics And Parkinsons Disease
The symptoms of Parkinsons disease appear to occur when the brain is no longer able to produce enough dopamine. Low dopamine levels in the brain can affect movement. It is not yet clear what role genetic factors may play in this process.
However, experts have identified specific genes in which changes appear to increase the risk of developing Parkinsons disease. The symptoms a person experiences may depend on their specific genetic changes.
Genetic changes can affect how mitochondria work. Mitochondria are the parts of a cell that produce energy. As they do this, they release byproducts commonly known as free radicals. Free radicals can cause cell damage.
Usually, cells can counter free radicals, but genetic changes can stop this from happening, and the free radicals can cause damage to dopamine cells.
Genetic changes can also lead to accumulations of a protein called alpha-synuclein in and around neurons throughout the brain. These accumulations are known as Lewy bodies, and the damage they cause can result in Lewy body dementia, which has links to Parkinsons disease.
Dopamine-producing nerve cells appear to be particularly susceptible to Lewy bodies, and some people develop both Parkinsons disease and Lewy body dementia.
Specific gene changes have specific outcomes. For example, SNCA affects the processing of alpha-synuclein, and PRKN impacts how mitochondria work.
The genetic changes involved in Parkinsons disease can be:
Where Are The Cures
So, given these hurdles, why are we pursuing genetics? Despite these limitations, we truly believe that the best possible route to treating this complex disease is to understand what goes wrong at the molecular level, and to use this knowledge to reverse, halt, or slow this process. Although the first genetic finding was made in PD more than 15 years ago, we still consider this early days in terms of therapeutic design. While there is a long way to go, we are clearly a lot further along the road to a cure than when we started, and the continued addition of new genetic findings can only take us further down this path.
Also Check: Parkinson Facial Expression
Is Parkinsons Disease Hereditary The Facts
Hereditary diseases are passed from parent to child through genes, but is Parkinson’s one of them? Even though Parkinson’s can be passed genetically, this is rare. Here are some facts to shed some light on the issue:
- Only 15% of people with Parkinsons reported having someone in the family with the condition.
- Having a parent with Parkinsons disease only increases your risk of getting Parkinsons by 3%.
- Most people with early-onset Parkinson’s disease are likely to have inherited it.
So is Parkinsons disease hereditary? Yes, Parkinsons disease can be genetic. But thats not to say you will inherit the Parkinsons disease gene if your parent or grandparent has the condition. Nor does it mean you wont develop it just because it doesnt run in your family. Parkinson’s can be traced to various gene mutations, but most of the time the cause is unknown.
Human Ipsc Studies Of Pd Highlight Converging Molecular And Cellular Pathways Across Genetic Subgroups
Our analysis of 385 iPSC-derived cell lines from 67 published studies reveals that many PD neuronal phenotypes are shared between genetically heterogeneous familial and sporadic patients . Notably, impairments in mechanisms involved in cellular waste recycling, mitochondrial function, neuronal morphology and physiology, and sensitivity to reactive oxygen species are most common across patient lines with varying genetic predispositions . The studies measured cellular phenotypes that occurred either spontaneously or in response to chemicals mimicking cellular aging and stress . It is important to note that the frequency of reported phenotypes in our meta-analysis may be biased because only few studies reported negative results ,,,,,,,,,,,,. In addition, most cell lines were not systematically phenotyped without prior hypothesis and thus, there is likely to be an ascertain bias in these phenotypes. Less hypothesis-driven multimodal or omics analysis will help to address such bias,,,,,,,,. Phenotypes caused by genomic predispositions allude to crosstalk and impairments in multiple pathways that act collectively to mediate selective degeneration of dopaminergic neurons in the substantia nigra and will be discussed in detail below.
Fig. 4: Phenotypic insights from iPSC studies of Parkinsons disease.
Read Also: What Are Early Warning Signs Of Parkinson’s Disease
