Role Of Lewy Bodies In Neurodegenerative Diseases
Lewy bodies represent abnormal assemblies of protein molecules inside the neurons in the brain and contribute to the pathology of Parkinson’s disease and other neurodegenerative diseases.
According to Gibb and Lees , Lewy bodies are the neuronal inclusions and are detected in substantia nigra in all cases of Parkinson’s disease. Moreover, the pattern of distribution of Lewy bodies in Parkinson’s disease is generalized. These are found in neurons in the dorsal vagal nucleus, locus coeruleus, nucleus basalis of Meynert, and hypothalamus. Additional regions of the brain including cerebral cortex, autonomic ganglia, and thalamus exhibited the presence of Lewy bodies in Parkinson’s disease . Neurodegeneration is the hallmark in most of the regions in which Lewy bodies have been detected in the brain.
Lewy bodies look like spherical aggregation inside the neurons. Lewy bodies are reported in the brain stem and cortex .
Lewy bodies have 830 m diameter and are made up of nearly 10 nm amyloidogenic fibrils like fibrillary -synuclein and neurofilaments . Lewy bodies have granular and fibrillar cores surrounded by a halo. A single neuron can have more than one Lewy body .
Two types of Lewy bodies have been described namely classical brainstem Lewy bodies and cortical Lewy bodies. The main morphological difference between the two types of Lewy bodies is that cortical Lewy bodies have diffuse outlines and are generally smaller in diameter without the presence of halo .
Potential Secondary Causes Of Fatigue
While current evidence suggests that fatigue is most commonly a direct result of PD pathology , it is important to ensure that other potential secondary causes of fatigue are not missed which may change treatment decisions and be entirely reversible. From the history, one may pick up on medications started around the time of fatigue onset , unusual symptoms in PD , or signs that should prompt further evaluations to determine if a comorbid condition is contributing to fatigue. As comorbid conditions are not always obvious on history or examination, I will also routinely check for orthostatic hypotension, anemia, and common metabolic conditions associated with fatigue at least once to ensure we are not missing a potentially reversible condition. Of note, these are rare contributors to fatigue in my practice, and although they have not been shown to significantly contribute to fatigue in PD in clinical trials, they do occur and treatment can result in dramatic improvements in some patients .
Ranjita Betarbet, J. Timothy Greenamyre, in, 2008
Pathophysiology Of Basal Ganglia Motor Control
Disruption of normal dopaminergic output from the substantia nigra interferes with the basal ganglia motor circuit. This circuit is involved in the facilitation of both voluntary and involuntary movement. The connections within the motor circuit are complex and not fully understood.Reference Wichmann, Watts and Koller28
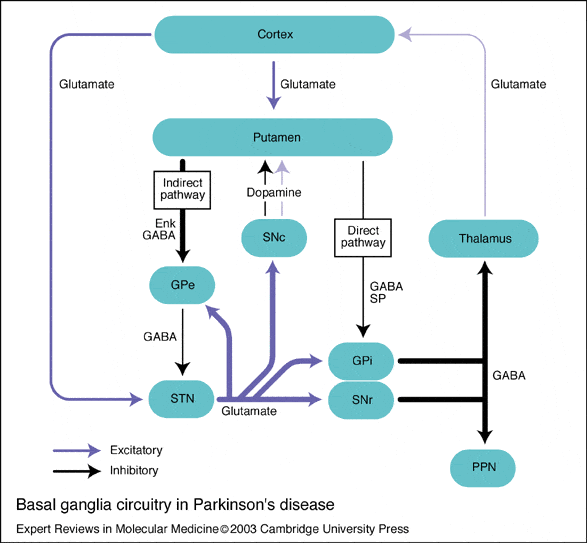
Figure 1. Basal ganglia circuitry normal and in Parkinson’s disease. Black arrows indicate inhibitory output, and white arrows indicate excitatory output. GPe, globus pallidus externa GPi, globus pallidus interna STN, subthalamic nuclei SNc, substantia nigra compacta SNr, substantia nigra reticulata VL, ventrolateral thalamus.
Striatonigral dopaminergic projections connect into the circuit via both a direct and an indirect pathway. The direct pathway involves D1 receptors and acts to reduce the inhibitory output from the globus pallidus interna , mediated by -aminobutyric acid . The indirect pathway involves D2 receptors. This pathway acts via the globus pallidus externa and the subthalamic nucleus, again to reduce inhibitory output from the GPi.Reference Gerfen, Engber, Mahan, Suzel, Chase, Monsma and Sibley29 In Parkinson’s disease, the brake on the GPi is diminished, resulting in increased inhibitory output into the thalamocortical motor circuit.
Read Also: How Does Caffeine Affect Parkinson’s Disease
Altered Firing Rates The Rate Model Of Parkinsons Disease
Chronic loss of dopamine results in significant changes in the firing rates of basal ganglia neurons, especially in the extrastriatal basal ganglia. Changes in the firing rates of MSNs have been difficult to identify, perhaps because MSNs are a heterogeneous population of neurons, and because dopamine loss affects corticostriatal transmission rather than directly altering the spontaneous activity of MSNs. Recent recordings in anesthetized 6-OHDA-treated rats, however, have shown less activity among direct pathway MSNs in parkinsonian animals than under normal conditions, while the spontaneous discharge and responses to cortical stimulation in indirect pathway MSNs are greater in dopamine-depleted animals than in normal ones . It is not clear whether this is specifically due to changes in the dopaminergic control of corticostriatal transmission, or whether the cortical or thalamic inputs themselves are altered. Of course, the loss of MSN spines, described above, may also impact the activity of these cells.
Besides the GABAergic system, glutamatergic transmission has also been evaluated. There is no consensus regarding changes in the binding or expression of striatal ionotropic glutamate receptors in parkinsonism , but these receptors appear to be down-regulated in the output nuclei of the basal ganglia in parkinsonian patients and in dopamine-depleted animals . This is, perhaps a compensatory response reflecting increased activity in the glutamatergic STN.
Parkinson’s Disease Pathology Aetiology And Diagnosis
Published online by Cambridge University Press: 08 June 2012
- Department of Medicine for the Elderly/Movement Disorders Clinic, Southern General Hospital, Victoria Infirmary, Glasgow, UK
- David A Stewart
- Department of Medicine for the Elderly/Movement Disorders Clinic, Southern General Hospital, Victoria Infirmary, Glasgow, UK
- *
- Address for correspondence: GJA Macphee, Consultant/Honorary Clinical Senior Lecturer, Department of Medicine for the Elderly/Movement Disorders Clinic ,
Don’t Miss: Does Resting Tremor Always Mean Parkinson’s
Selective Vulnerability Of The Nigrostriatal Dopamine Neuron
In PD, dopamine neurons within the substantia nigra are considered a selectively susceptible population of cells, whereas the adjacent dopamine neurons of the ventral tegmental area are much more resistant to degeneration. The vulnerability of the nigrostriatal neurons is due to several factors, including their unique anatomy, physiology, bioenergetic profile, and neurochemistry . First, in rat brain, the length of the axon arbor of a single nigrostriatal neuron is up to 80 cm in humans, this is estimated to be 4 m! In the rat, a single nigrostriatal neuron makes 100000240000 synapses in the striatum. In humans, it is estimated that a nigrostriatal neuron makes 10000002400000 synapses. By contrast, the VTA neuron makes about 10-fold fewer synapses and therefore has a much lower bioenergetic demand. Further compounding the bioenergetic demand of the nigrostriatal neuron is the fact that their axons are unmyelinated. Thus, propagation of each action potential and subsequent repolarization requires much more energy than if the fibers were myelinated.
Impact Of Parkinson’s Disease Related Genetics On Astrocyte Function
There are monogenic mutations identified in 20 genes that have been implicated in the pathogenesis of PD . Interestingly, a study by Zhang et al. compared the transcriptome of human astrocytes to neurons, and found upregulation of some of these monogenic mutations in astrocytes was to a similar level and sometimes higher than that of neurons . This would strongly support the potential contribution of astrocytes to the pathogenesis of these familial forms of PD. Altered levels of these genes lead to many changes in astrocyte function including impaired glutamate uptake, liposomal homeostasis, lysosomal, and mitochondrial dysfunction and inflammatory response .
Table 1. The role of genes that are causative in Parkinson’s disease pathogenesis and their implications in astrocytes.
DJ-1/PARK7
PARK2 and PINK1
SNCA
LRRK2 and GBA
Other Parkinson’s Disease Risk Genes
Recommended Reading: Exercise And Parkinson’s Disease
Circuit Anatomy Of The Basal Ganglia
Parkinsonism is considered to result primarily from abnormalities of basal ganglia function. The basal ganglia include the neostriatum , the external and internal pallidal segments , the subthalamic nucleus , and the substantia nigra with its pars reticulata and pars compacta . They participate in anatomically and functionally segregated loops that involve specific thalamic and cortical areas. These parallel circuits are divided into motor, associative and limbic loops, depending on the function of the cortical area involved . The thalamic components of these circuits are largely separate from those engaged by cerebellar outflow pathways .
Striatum and STN receive glutamatergic afferents from specific areas of the cerebral cortex or thalamus, and transfer the information to the basal ganglia output nuclei, GPi and SNr. The projections between the striatum and GPi/SNr are divided into two separate pathways, a direct connection, and an indirect projection, via the intercalated GPe and STN. Output from GPi/SNr goes largely to the ventral anterior and ventrolateral nuclei of the thalamus , which, in turn, project back to the cerebral cortex. Lesser basal ganglia projections reach the intralaminar centromedian and parafascicular thalamic nuclei and brainstem structures such as the superior colliculus, pedunculopontine nucleus , and the reticular formation.
Changes In Brainstem Activity
Our understanding of the pathophysiologic changes that occur in areas outside of the basal ganglia-thalamocortical loops remains rudimentary. However, there is evidence that abnormalities in brainstem regions, specifically the PPN, may be involved in the development of some of the core signs of parkinsonism. The PPN is tightly connected to the basal ganglia . Animal studies demonstrate that stimulation of the PPN area increases movement , while inhibition or lesioning of the PPN decreases it . In MPTP-treated monkeys, akinesia is reduced by injections of a GABA receptor antagonist into the PPN or by low-frequency electrical stimulation of the PPN area . Studies in 6-OHDA-treated rats have suggested that PPN activity is increased in the dopamine-depleted state , and that lesions of the PPN in 6-OHDA treated rats reduce some of the discharge abnormalities in STN and SNr .
Caution is necessary in the interpretation of the effects of surgical PPN interventions in animals or humans, because this nucleus is heterogeneous, it is not well demarcated from surrounding brain regions, and the spread of current of drugs from the site of intervention to surrounding areas cannot be excluded . The optimal stereotactic target of surgical interventions in humans has not been determined, and the electrophysiologic properties of the PPN and nuclei around it are not fully defined, rendering electrophysiologic targeting of the surgical procedures more difficult than it is in the basal ganglia
Also Check: Is Parkinson’s Considered A Neurological Disease
Animal Preparation And Transplantation
All experimental procedures were approved by the University of South Florida Institutional Animal Care and Use Committee and followed the ARRIVE 2.0 guidelines . All investigators were blind to the treatment condition until after completion of all data analyses. This study used 14 months-old male C57BL/6NJ and C57BL/6N-Tg 15Mjff/J mice . The Tg mice overexpressed the wild type human -synuclein, with validated phenotypic PD-like progressive nigrostriatal dopamine depletion and motor deficits . All mice were included in the study, had free access to food and water, and housed under normal conditions . Mice were randomly assigned by a staff not involved in the study to one group: Wt Con , Wt+P , Wt + hUCB+P , Tg Con , Tg + P and Tg+hUCB+P . 0.4×106 hUCB cells in 50L of hUCB plasma or only 50L of hUCB plasma were intravenously injected using the jugular vein to the treatment group . Sample size ensured adequate power to detect 25% treatment effect size.
Fig. 6: Treatment timeline.
Behavioral tests at baseline were performed on Day 0 prior to transplantation. On Day 1, intravenous transplantation with P or hUCB+P was performed. Behavioral tests were conducted again on day 1, 3, and 7 post transplantation. Animals were sacrificed on day 7 for brain and gut for fluorescent insitu hybridization and immunohistochemistry to assess PD histopathology.
You can also search for this author inPubMed
Deep Brain Areas Brain Diseases And Lutd
Substantia nigra pars compacta
Degeneration of substantia nigra pars compacta leads to an altered fronto-nigro-striatal, dopamine D1-GABAergic direct pathway , which is thought to be an anatomic substrate of DO in Parkinson’s disease . No focal diseases affecting substantia nigra and causing LUTD have been reported.
Striatum
Striatum is the major site of lesion in Huntington’s disease, where GABAergic cell loss occurs. Degeneration of striatum may also lead to altered fronto-nigro-striatal pathway in experimental animals . However, in contrast to common bladder dysfunction in Parkinson’s disease, bladder dysfunction in Huntington’s disease, a degenerative disease affecting both striatum and the cerebral cortex, is not recognized, but has been reported by Wheeler et al. . Four of six patients in their study showed DO. No focal diseases affecting striatum and causing LUTD have been reported.
Hypothalamus
Cerebellum
The above clinical and experimental studies suggest that the cerebellum has an inhibitory influence on the micturition reflex. Loss of the cerebellum’s inhibition may have led to DO in patients with cerebellar lesion.
Brainstem
Midbrain tegmentum
Pontine tegmentum
Pontomedullary basis: the medullary raphe area
Read Also: Do Parkinson’s Tremors Stop When Sleeping
What Causes Parkinsons Disease
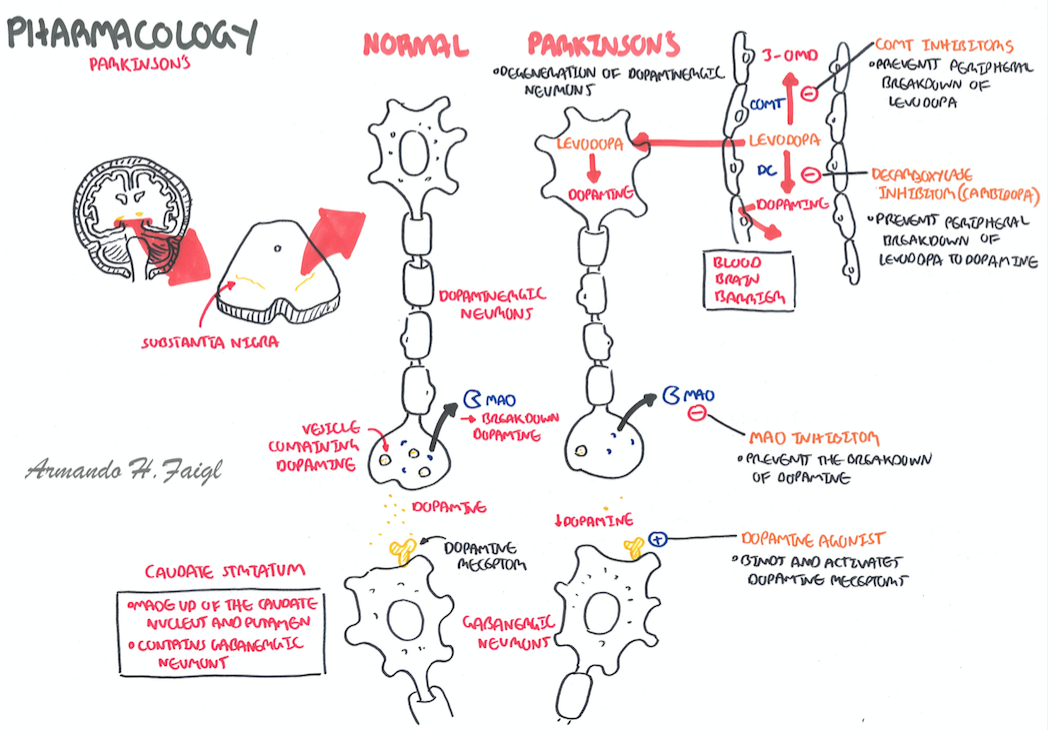
The most prominent signs and symptoms of Parkinsons disease occur when nerve cells in the basal ganglia, an area of the brain that controls movement, become impaired and/or die. Normally, these nerve cells, or neurons, produce an important brain chemical known as dopamine. When the neurons die or become impaired, they produce less dopamine, which causes the movement problems associated with the disease. Scientists still do not know what causes the neurons to die.
People with Parkinsons disease also lose the nerve endings that produce norepinephrine, the main chemical messenger of the sympathetic nervous system, which controls many functions of the body, such as heart rate and blood pressure. The loss of norepinephrine might help explain some of the non-movement features of Parkinsons, such as fatigue, irregular blood pressure, decreased movement of food through the digestive tract, and sudden drop in blood pressure when a person stands up from a sitting or lying position.
Many brain cells of people with Parkinsons disease contain Lewy bodies, unusual clumps of the protein alpha-synuclein. Scientists are trying to better understand the normal and abnormal functions of alpha-synuclein and its relationship to genetic mutations that impact Parkinsons andLewy body dementia.
Neuropathology Of Parkinsons Disease
Macroscopically, the brain in idiopathic PD is often unremarkable with mild atrophy of the frontal cortex and ventricular dilation in some cases. The main distinctive morphological change in the PD brain is observed in transverse sections of the brainstem, where almost all cases present with loss of the darkly pigmented area in the substantia nigra pars compacta and locus coeruleus. This pigmentation loss directly correlates with the death of dopaminergic neuromelanin-containing neurons in the SNpc and noradrenergic neurons in the locus coeruleus . Cell death in the SNpc is mostly restricted to a specific group of neuromelanin-containing dopaminergic neurons, namely the A9 neurons, while other neuronal and glial cell types are largely spared .
Coronal section at the level of the substantia nigra pars compacta in a control and a PD brain stained by hematoxylin and eosin. In both sections, the dark brown cells are the neuromelanin-containing dopaminergic neurons.
Recommended Reading: Does Parkinson Disease Qualify For Disability
Support For People Living With Parkinsons Disease
While the progression of Parkinsons is usually slow, eventually a persons daily routines may be affected. Activities such as working, taking care of a home, and participating in social activities with friends may become challenging. Experiencing these changes can be difficult, but support groups can help people cope. These groups can provide information, advice, and connections to resources for those living with Parkinsons disease, their families, and caregivers. The organizations listed below can help people find local support groups and other resources in their communities.
Read A Brief Summary Of This Topic
parkinsonism, a group of chronic neurological disorders characterized by progressive loss of motor function resulting from the degeneration of neurons in the area of the brain that controls voluntary movement.
Parkinsonism was first described in 1817 by the British physician James Parkinson in his Essay on the Shaking Palsy. Various types of the disorder are recognized, but the disease described by Parkinson, called Parkinson disease, is the most common form. Parkinson disease is also called primary parkinsonism, paralysis agitans, or idiopathic parkinsonism, meaning the disease has no identifiable cause. This distinguishes it from secondary parkinsonism, a group of disorders very similar in nature to Parkinson disease but that arise from known or identifiable causes. The onset of Parkinson disease typically occurs between the ages of 60 and 70, although it can occur before the age of 40. It is rarely inherited. Parkinson disease often begins with a slight tremor of the thumb and forefinger, sometimes called pill-rolling, and slowly progresses over 10 to 20 years, resulting in paralysis, dementia, and death.
Also Check: What Stage Of Parkinson’s Is Michael J Fox In
Microglia And Their Role In Parkinson’s Disease
Microglia are the resident immune cells of the CNS. They originate in the yolk sac where they develop from early myeloid precursor cells. During embryonic development, primitive microglia migrate into the developing neural tube where they proliferate and populate the CNS . Due to the BBB, microglia lead a relatively sheltered existence compared to peripheral macrophages, although their functions remain the same. Their role is to continuously survey the microenvironment and respond to both physiological and pathological changes. In their capacity as the first line of defence in the CNS, they identify and remove unwanted material such as cellular debris.
A Potential Role For T Cells In Parkinson’s Disease
In the last decade there has been mounting evidence of a role for T cells in the pathogenesis of PD . Despite their important role as part of the adaptive immune system there is little evidence of the involvement of B cells in PD. Brochard et al., identified both CD8+ and CD4+ T cells but not B cells or natural killer cells in the post-mortem brain tissue of PD patients . The presence of both CD8+ and CD4+ T cells was also evident in the MPTP and -syn overexpressing mouse models of PD . However, Theodore et al., observed both infiltrating B and T cells following injection with -syn overexpressing adeno-associated viral vector into the SN of mice . The differences with respect to the possible role of B cells in these studies may be attributed to the study model and human PD tissue vs. animal model, however more research is required before a definitive conclusion can be drawn.
Table 3. Evidence for the role of T lymphocytes in Parkinson’s disease.
The Role of MHC and Antigen Presentation in Parkinson’s Disease
Mitochondrial Antigen Presentation in Parkinson’s Disease
Th1 Cells in Parkinson’s Disease
Th17 Cells in Parkinson’s Disease
Th2 Cells in Parkinson’s Disease
T Cell Regulation in Parkinson’s Disease
You May Like: How Long Can One Live With Parkinson’s Disease
