Clinical Characteristics Of Mutation Carriers And Comparison Of Lrrk2 Gly2019ser Mutation Carriers With Individuals With No Known Pd Mutations
Co-segregation analyses identified 193 PD patients as mutation carriers: 151 with LRRK2 Gly2019Ser and five with Arg1441His mutations, 29 with SNCA and eight with VPS35 Asp620Asn mutations.
SNCA
The clinical characteristics of the 29 PD patients carrying either SNCA rearrangements or missense mutations are shown in Table 3. All but three of the families concerned originated from France. The remaining three families, originating from Italy, Turkey and Morocco, all had SNCA duplications. Within this cohort, SNCA duplications were the most frequent mutation identified , followed by the Ala53Thr mutation . Disease onset occurred earliest in patients with the Ala53Thr mutation .
Table 3. Summary of the clinical data for patients carrying SNCA, VPS35, and LRRK2 Arg1441His mutations identified in this study.
VPS35
Five of the eight PD patients carrying VPS35 Asp620Asn mutations have been described before . The three newly genotyped patients were relatives of patient 838006 . Briefly, patients carrying VPS35 mutations had features similar to those with idiopathic PD, with a mean AAO of ~57 years : all patients presented the classical triad, with akinesia as the predominant symptom at onset , but a much lower frequency of tremor as an initial symptom , a good response to levodopa , with <37% of those treated developing dyskinesias and motor fluctuations, and a low rate of dysautonomia , with no cognitive or neuropsychiatric symptoms or atypical signs.
LRRK2
Causes Of Parkinson’s Disease
| This article may be too technical for most readers to understand. Please help improve it to make it understandable to non-experts, without removing the technical details. |
Parkinson’s disease is a degenerative disorder of the central nervous system. Most people with PD have idiopathic Parkinson’s disease . A small proportion of cases, however, can be attributed to known genetic factors. Other factors such as environmental toxins, herbicides, pesticides, and fungicides, have been associated with the risk of developing PD, but no causal relationships have been proven.
Are Genes Responsible For Monogenic Disorders Also Susceptibility Factors
Associations detected by screening candidate genes in controls and patients cannot always be replicated in follow-up studies, and few candidate genes were confirmed in meta-analysis, because of potential biases and confounding factors, including population stratification, small sample size, misclassification and/or inappropriate statistical methods. Polymorphic variants in SNCA and LRRK2 genes, and heterozygous mutations in the GBA gene, however, have been validated as genetic susceptibility factors .
Nucleotide polymorphisms located close to the promoter region and throughout SNCA have been associated with sporadic PD, although much of the data is equivocal . Rep1 , a mixed nucleotide repeat, 10 kb upstream of the translational start of SNCA , has been confirmed as a risk factor , and synergy between an SNCA variant and a polymorphism in microtubule-associated protein tau , each of which increases the risk for the development of PD, has been detected . The combination of risk genotypes in SNCA and MAPT doubles the risk of PD, further supporting the notion that the related pathways contribute to neurodegenerative diseases . The risk associated with Rep1 does not interact, however, with herbicide exposure, an independent risk factor in PD .
Also Check: Parkinsons Disease Gene
Molecular Diagnostic Testing In Pd
Molecular diagnostic testing is currently available for PRKN , PINK1 , DJ-1 , and LRRK2 . However, molecular testing and genetic counseling is challenging in PD patients and their families because of the varied patterns of inheritance that has been observed. Clear review of the family history and clinical symptoms is essential in prioritizing the molecular tests. Importantly, clinical recommendations are not altered based on the presence or absence of a particular molecular mutation. Therefore, molecular diagnostic testing is not essential in the current management of PD patients.
For PD patients with an early onset of disease, it has been estimated that 50% of those with onset before age 40 have a mutation in PRKN, PINK1, or DJ-1. However, given the rarity of PINK1 or DJ-1 mutations, initial testing of PRKN would seem to be the most cost effective strategy. Based on the distribution of age of onset of patients with PRKN mutations, it has been recommended that those patients with onset prior to age 40 be considered for screening for PRKN mutations. Although mutations have been found in those who onset above age 40, the rate is quite low and therefore testing is not likely to be cost effective.
In patients with early onset PD who have been tested for PRKN and found to be negative, it may be appropriate to consider screening PINK1 and DJ-1. However, mutations in both of these genes are much less frequent than those in PRKN.
Genetic Principles And Exceptions Thereof In Familial Pd
The majority of PD cases are sporadic, i.e., only about 10% of patients report a positive family history . Out of the six genes unequivocally linked to heritable, monogenic PD, mutations in SNCA , and LRRK2 are responsible for autosomal-dominant PD forms, and mutations in Parkin , PINK1 , DJ-1 , and ATP13A2 are accountable for PD that displays an autosomal recessive mode of inheritance.
In general, the inheritance patterns of human disorders are identified by examining the way the disorders are transmitted in the family of the index patient. Such a pedigree analysis requires a careful assembly of the disease records of the family members over several generations, and if possible, examination and sample collection from affected and unaffected individuals from the pedigree. All of the currently known monogenic PD forms are autosomal , which means that they are linked with regions on autosomes .
Pedigree of a PD family that comprises affected members with and without the LRRK2 p.G2019S mutation. Five mutation carriers are unaffected, showing reduced penetrance, two mutation carriers are affected with dystonia, showing variable expressivity, and one affected family member does not have the p.G2019S mutation in LRRK2. Black symbols – affected individuals; white symbols – unaffected individuals; half-filled symbols – individuals with dystonia; + – mutation carriers.
Also Check: What Is The Life Expectancy Of Someone With Parkinson’s Disease
Autosomal Dominant Genetic Features
People have two copies of each gene. In autosomal dominant inheritance, a child can inherit either a healthy gene or one that is not working correctly. They will have a 50% chance of inheriting a faulty gene.
Autosomal dominant genes that have associated with Parkinsons disease include:
- SNCA, or PARK1
- DJ-1
- PRKN
It is important to note that inheriting any of the genes that scientists have identified as being related to Parkinsons disease does not necessarily mean that a person will develop the condition.
Monogenic Variants Of Parkinson Disease
Disease phenotypes associated with the PARK1–9 chromosomal loci follow a typical MENDELIAN pattern of inheritance , whereas PARK10 and PARK11 represent susceptibility loci with as yet undefined modes of transmission. In Mendelian genetics, the relationship between genotype and phenotype is not always readily apparent; for example, as illustrated in Figure 2, single heterozygous mutations in ‘recessive’ genes can act as susceptibility factors, thereby appearing pseudodominant. Similarly, dominant forms can present in a pseudorecessive fashion, and heritability should be suspected even in early-onset patients with a negative family history . An apparent lack of heritability might be explained by small family size, nonpaternity, adoption, variable clinical characteristics, reduced PENETRANCE, or de-novo mutations. Conversely, because sporadic PD is a relatively common condition, familial PD might be phenocopied by an occurrence of sporadic PD in a pedigree with a well-established genetic background of the disease.
Figure 2.
Recessively Inherited Parkinson Disease: Probable Loss-of-Function Mechanism
Recently, two biochemical modifications of Parkin were identified in cellular studies and human brain specimens. These data indicated that reduced E3-ligase activity of the wild-type Parkin protein could also occur as a result of the principal pathogenetic process that is responsible for the development of sporadic PD.
Figure 3.
Don’t Miss: How Long Do You Live With Parkinson’s
Genetic Testing And Parkinsons Disease
After reading about the different genes related to Parkinsons, you may wonder if you have any of these genes. However, Dr. Tropea does not recommend going out and getting a genetic test from a direct-to-consumer brand like 23andme.
Theres no easy way to find out if youre genetically at risk. Its probably a big waste of money, he said.
If youre going to get a genetic test, its better to go to the professionals. UPenns MIND Initiative conducts genetic tests for the GBA and LRRK2 genes in Parkinsons patients. The Parkinsons Foundations PDGENEration program also provides free genetic testing and genetic counseling for Parkinsons patients.
Causes Of Parkinson Disease
Parkinson disease results from complex interplay of non-genetic and genetic factors. However, genetic factors are increasingly recognized as causative.
Typically Parkinson disease occurs in only one family member ; less commonly it occurs in several family members . Historically, about 15% of individuals with Parkinson disease have a positive family history of PD.
An estimated 5%-10% of all Parkinson disease is attributed to pathogenic variants in single genes . In addition to these variants, other genetic and environmental factors â known and unknown â may contribute to overall risk .
You May Like: Parkinsons Weakness
Risk Factors Identified By Genome
Genome-Wide Association studies look at markers across complete sets of DNA to find genetic variations associated with a particular disease. The goal in identifying genetic associations is to provide targets for additional research to detect, treat, and prevent diseases. Genetic variations that have been found in GWA studies that are associated with PD include:
- MAPT
Identification Of New Genes And Risk Factors For Pd
New PD-linked genes or PD risk factors can be identified by gene mapping or candidate gene approaches. Gene mapping in human diseases is the localization of genes underlying the clinical phenotypes of the disease on the basis of correlation with DNA variants , without the need for prior hypotheses about biological function. Genetic mapping methods include linkage analysis and genome-wide association studies. Alternatively, based on their known function, levels of expression, or mode of interaction , some genes can be considered plausible candidates, and as such, tested for in cohorts of patients.
You May Like: Essential Oils Parkinsons
When To See A Doctor About Parkinsons
There isnt one specific test to diagnose Parkinsons disease. Doctors will usually evaluate your symptoms and perform several tests to determine if you have the condition. If you notice the following early warning signs, then you should see a doctor.
The early warning signs of Parkinsons disease include:
A The Genetic Contribution To Pd Has Been Greatly Underestimated
Genetic research in the last decade, in particular the mapping and the subsequent cloning of genes that cause heritable forms of the disorder, has shown that PD is not a single clinical entity, but rather a heterogeneous group of diseases with different associated pathologies and a variable spectrum of clinical signs and symptoms. Thus some familial forms include atypical clinical features, such as young-onset, onset with dystonia, or the early occurrence of dementia or dysautonomia. However, despite excitement about these recent advances, only 510% of patients with a clinical picture of PD carry a mutation in one of the known genes that cause autosomal dominant or recessive forms of the disease. The lack of a clear family history in monogenic forms can result from recessive inheritance, a censored effect that may occur for multiple reasons, including death as the most common event prior to the observation of the disease or loss of follow-up, reduced penetrance, or a dominant de novo mutation. Alternatively, the disorder may result from a genetic predisposition to an environmental toxin, or to a combination of several genes that each increases the risk of the disease to only a modest extent.
Recommended Reading: What Is The Life Expectancy Of Someone With Parkinson’s Disease
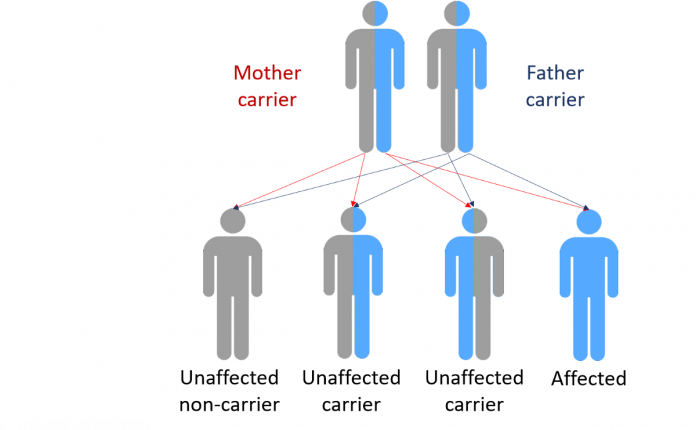
Recessive And Dominant Genetic Conditions
Eye colour is, in reality, a little more complex than this, but some diseases work in this way. For instance, cystic fibrosis is a recessive genetic condition that only affects people if they inherit two copies of the faulty gene.
As we can see in the diagram, if a person has one faulty gene they are not affected but are a carrier.
Should a carrier have a child with another carrier that child would have a 1 in 4 chance of inheriting cystic fibrosis.
Family trees of those with recessive condition tend to have characteristic patterns based on these probabilities, but can be very complicated as it is hard to identify unaffected carriers.
Human diseases can also be caused by dominant genes. One example of this is Huntingtons where inheriting a single version of the faulty gene is sufficient to cause the condition. The pattern within a family tree left by dominant genetic traits or conditions is somewhat easier to identify at least half of all the children of someone with a dominant inherited condition, who has children with someone without the condition, will also have the condition.
Why Genetic Testing For Parkinsons Disease Is Complex:
- There are many genes that are associated with the development of PD. This list continues to grow as more genes are discovered. Testing of only some of these genes is available in commercial labs.
- The majority of people with PD, even those with a family history of PD, do not harbor one of these identified abnormal genes. The genetic contribution to PD in these people is yet to be discovered.
- For a particular gene there may be a number of different mutations associated with disease, some of which are more common than others. Commercial testing may identify only the most common of the mutations, and therefore not capture everyone who carries a disease-causing mutation.
- Conversely, only particular mutations in a gene may be associated with disease. Commercial testing may identify changes in a gene that may not have clinical consequences. This can be confusing for patients who even after genetic testing may not know whether they harbor a disease-causing mutation.
- Different mutations can be enriched in different ethnic populations. For example, Ashkenazi Jews and North African Berbers have an increased risk of carrying Leucine rich repeat kinase 2 mutations. Glucocerebrosidase mutation frequency also varies greatly with ethnicity and is also increased among Ashkenazi Jews.
In addition to the above, it is important to realize that not all genes associated with PD contribute to disease in the same way:
Read Also: Stages Of Parkinson’s Disease Life Expectancy
Who Should Consider A Genetic Test For Parkinsons
There are two groups of people who might consider getting genetic testing and we will discuss each group separately.
Genetic testing for PD is a common request and a number of commercial labs perform panels of genetic testing for PD. You may ask: How can I test myself for Parksinons? Whether youre considering getting a genetic test through your doctor, or performing one at home, its important to note that at-home test dont map the entire gene for mutations. Genetic testing through your doctor will test for GBA, PARK7, SNCA, LRRK2, parkin and PINK1.
Both groups are faced with two questions: Should I get genetic testing? And if so, what should I do with the results? Before we address these two questions, we need to learn more about the complexity of genetic testing in PD.
Genetics Testing And Research
Although there may be no direct benefit to you at the present time, the results of genetic testing can help further Parkinsons research by allowing scientists to better understand the disease and consequently develop new treatments. For example, a mutation in the gene that codes for the protein alpha-synuclein leads to a specific type of familial Parkinsons disease. Although this mutation only accounts for a small percentage of cases, knowledge of this mutation has had broader effects. The study of this genetic mutation led to the discovery that alpha-synuclein clumps together to form Lewy bodies which have been consistently found in the brains of all individuals with Parkinsons disease not just those with the SNCA mutation. Thus, one gene mutation has led to a critical finding in the field of Parkinsons research.
Genetics testing is a very personal decision but a cautionary note: anytime that genetic testing is considered, particularly in a disease condition where there is no change in treatment based on genetic findings, it would be my recommendation to see a genetics counselor to discuss the impact this information will have on you the patient and your family.
Don’t Miss: Will There Ever Be A Cure For Parkinson’s
New Genes For Recessive And X
Compared to monogenic dominant PD and to the well-established recessive early-onset PD genes PARK2, DJ-1, and PINK1, the newly identified recessive forms appear more complex both for clinicians and researchers. The clinical picture of the newly identified recessive forms is often more severe and multifaceted. However, in a few instances, there appears to be a genotype-phenotype correlation where mutations that lead to pronounced alteration of normal protein function cause a complex disorder with severe additional neurological or neuropsychiatric impairment, often from birth, and juvenile Parkinsonism, whereas mutations with a milder effect on the protein cause Parkinsonism with fewer atypical features. Table 2 summarizes currently known and putative genes for recessive and X-linked PD or Parkinsonism.
Table 2 Monogenic causes for autosomal recessive or X-linked Parkinsons disease or atypical juvenile Parkinsonism
E Lessons From Neuropathology
PD with autosomal recessive inheritance differs generally from idiopathic PD, although cases with a clinical course indistinguishable from that of the typical disease have been reported. Autosomal recessive PD is characterized by 1) early disease onset, in most cases before age 40; 2) benign, slowly progressive disease course; 3) excellent response to levodopa but early levodopa-induced dyskinesias; and 4) minimal cognitive decline, minimal dysautonomia. It is consistent with neurodegeneration mainly restricted to the dopaminergic neurons of the SNc, as confirmed by the neuropathological analyses of the few cases that have come to autopsy. The only autosomal recessive forms of PD that have been examined post mortem for brain pathology are parkin- and, most recently, PINK1-linked diseases.
In light of all these observations, it becomes increasingly clear that the subdivision of parkinsonian syndromes into two physiopathological groups with distinct neuropathology, Lewy body-PD-related autosomal dominant forms and recessive parkinsonism without synucleinopathy, is to a certain degree artificial and may be misleading .
Don’t Miss: Is Parkinson Fatal