Who Should Get Genetic Testing
Two groups might consider getting genetic testing, according to Gilbert:
- People with Parkinsons who want to know if they have a mutation they may pass along to their children
- Children and siblings of family members with Parkinsons who want to determine their genetic risk for the disease
Right now its not standard of care for everyone with Parkinsons to get genetic testing, she says. The likelihood that were going to find one of these mutations that is known already is small, and even if you have a mutation associated with Parkinsons, it doesnt mean that youre going to get the disease.
So, at this point, the value of getting tested depends on the individual. Doctors can provide this type of genetic evaluation, or people may turn to direct-to-consumer genetic testing, such as 23andMe. These tests, however, can be limited.
You have to be careful with those panels because theyre not very comprehensive, says Gilbert. They may test for only one or two gene variations.
Currently, 23andMe analyzes DNA from spit samples for a variant in LRRK2 and a variant in the GBA gene associated with the disorder. The company makes it clear that the exam does not diagnose the disease, and there are many other mutations to consider.
Parkinsons patient Paul Cannon, PhD, who works for 23andMe as its Parkinsons research community manager, took the test and found that he had neither of the genetic variations.
Predicting Population Risk Of Pd Onset
Combined with incidence rates from epidemiological studies, PHSs can be used to calculate genetically stratified estimates of absolute disease risk across the age spectrum., We defined the age-dependent baseline risk based on epidemiological incidence rates by age group from a comprehensive 2017 report on âThe Incidence and Prevalence of Parkinson’s in the UK,â representing to our knowledge the largest and most recent data source to provide the figures of interest. As incidence rates were reported for 5-year intervals, we let values represent the midpoint of each interval and used one-dimensional interpolation to estimate annualized incidence rates. Hazard ratios of PHS percentile strata were used to visualize the influence of polygenic risk on incidence curves and recalculate stratified âinstantaneousâ risk across age groups, applying sample weight correction to account for different caseâcontrol proportions in the sample sets as detailed in a previous report.
How Often Does Parkinsons Run In The Family
Most Parkinsons cases have no connection to a genetic cause, but scientists have found that some gene mutations can heighten an individuals risk. Researchers believe that a better understanding of these genes may improve ways of identifying and treating the illness.
The National Institute of Neurological Disorders and Stroke reports that an estimated 15 to 25 percent of people with Parkinsons have a family history of the disorder. The Michael J. Fox Foundation for Parkinsons Research estimates that about 10 percent of cases are linked with a genetic cause.
Parkinsons doesnt stand out as a hereditary disease over and above any other chronic diseases that people deal with, says Rebecca Gilbert, MD, PhD, chief scientific officer for the American Parkinson Disease Association in New York City. But if you have a parent with Parkinsons disease, you have about a fourfold greater risk over the general population.
Still, that risk is relatively small. About 1 percent of the population over 60 has Parkinsons, according to the Michael J. Fox Foundation, and that number rises to about 4 percent for those who have a mother or father with the illness, according to Dr. Gilbert. The overall message is: Just because you have a gene linked to Parkinsons does not mean you will get the disease.
Read Also: How To Use Hemp Oil For Parkinson’s
Evaluating The Performance Of Sex
In previous studies of AD, training and testing the PHS model in sex-matched data sets have been shown to significantly improve performance, indicating that genetic risk interacts with sex, at least for a relevant subset of common susceptibility loci. To evaluate this possibility in PD, we repeated the same workflow for model training and testing in the same data sets using male-only and female-only subsets of the data, respectively.
Some Patients May Not Be Eligible To Take Part In The Free Genetic Testing Program
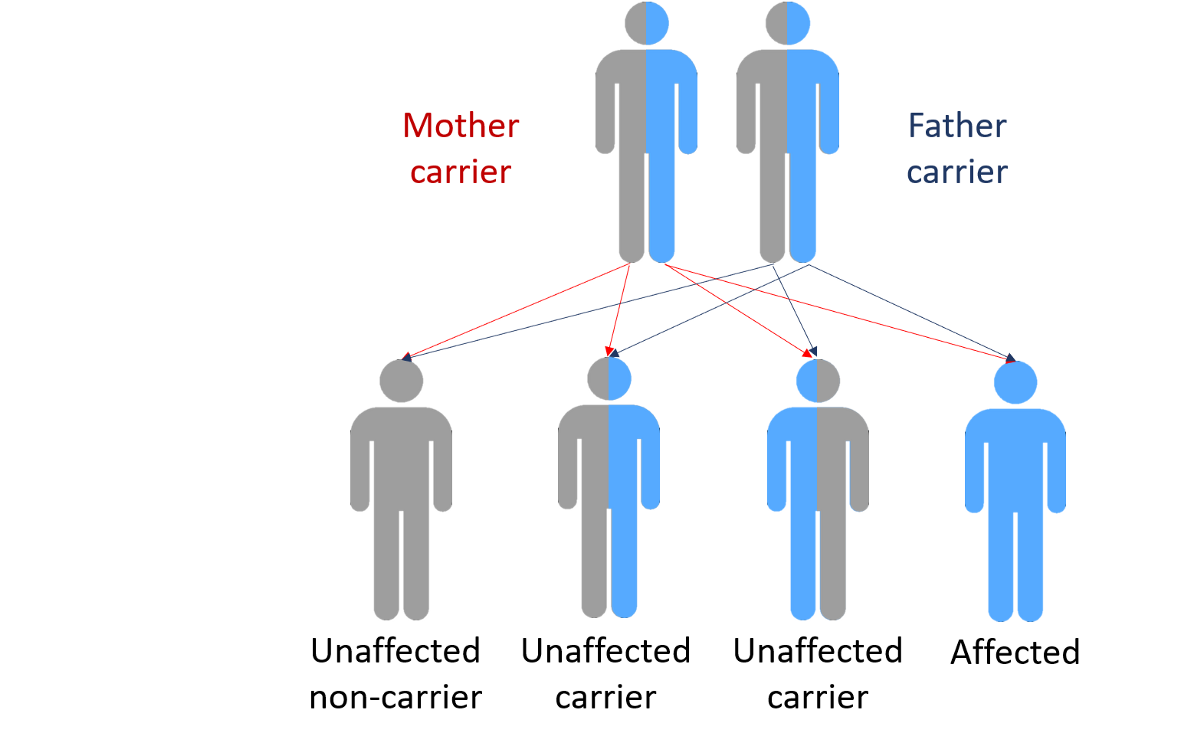
To speed up recruitment for a natural history program we are first focusing on patients who have a higher likelihood of testing positive for the mutated gene from the free genetic testing program. If a patient does not meet the criteria now, we will offer to contact them again should we open up the screening program to a wider population of Parkinsonâs patients.
Also Check: What Is The Life Expectancy Of Someone With Parkinson’s Disease
New Genes For Dominant Pd
Since 2012, mutations in DNAJC13, CHCHD2, TMEM230, and RIC3 have been reported as new causes for monogenic dominant PD. The following sections provide a review of the available reports on the clinical picture and genetic information. All of these discoveries are recent, and it is today not definitely proven that mutations in these genes cause PD. The present evidence appears most robust for a causative role of CHCHD2 mutations in PD because of the description of more than one family with co-segregation of clinical phenotype and genotype, but less robust for DNAJC13 and TMEM230, because these mutations were found in only one, albeit large, pedigree, and least robust for RIC3 that has only been reported from one less extensive family. Table 1 provides an overview over presently known genes for dominant PD, which generally cause medium- to late-onset Parkinsonism or PD, for most genes with few or no additional features.
Table 1 Monogenic causes for autosomal dominant Parkinsons disease
Predicting Risk Progression And Defining Etiological Subtypes Of Disease
Individually GWA identified loci confer relatively small amounts of disease risk however, the use of polygenic risk scores affords the ability to attribute a total known genetic risk score to an individual by summing their collective genetic risk. To date, the PRS reveals that, collectively, the 90 susceptibility loci confer considerable risk for disease, with those in the top decile of genetic risk being 6-fold more likely to have PD than those in the lowest decile of genetic risk . Additionally, by creating a composite risk score for PD diagnosis that combines the cumulative effect of genetic risk variants as well as the presence or absence of anosmia, age, sex, and family history, the ability to predict individuals at high risk for PD is remarkable, showing an AUC sensitivity of ~ 83.4% and specificity of ~ 90% .
Research focused on age at onset disease modifiers is one area where consistent effort has been seen. The largest PD age at onset GWAS to date included data from > 25K cases and identified two GWAS significant signals one at SNCA and the other was a protein-coding variant in TMEM175, both of which are known PD risk loci. Notably, these results showed that not all PD risk loci influence age at onset and therefore suggest the idea that risk and onset might operate through mechanisms that do not completely overlap.
Recommended Reading: Stage 4 Parkinson’s Disease Life Expectancy
Epigenomic Alterations Linked With Pd In Patient
The ability to capture unique epigenomic alterations associated with PD remains an important challenge. Reprogramming fibroblasts to iPSCs may erase age-associated and naive epigenetic signatures which could contribute to sporadic PD pathophysiology. However, an epigenetic phenotype was reported in iPSC-derived PD patient neurons,. Neuronal lines derived from LRRK2 and sporadic patients exhibited epigenomic alterations when compared with healthy controls. Hypermethylation was prominent in gene regulatory regions associated with the downregulation of transcription factors FOXA1, NR3C1, HNF4A, and FOSL2. Interestingly, LRRK2 mutant and sporadic PD patient neurons shared similar methylation patterns, which were absent in the original donor fibroblasts. A spontaneous increase in the number of DNA strand breaks and genomic damage in PD patient-derived neurons could indirectly impact genomic regulation.
What Determines Who Gets Parkinson’s Disease
In most cases inheriting a non-working copy of a single gene will not cause someone to develop Parkinson’s disease. We believe that many other complicating factors such as additional genes and environmental factors determine who will get the condition, when they get it and how it affects them. In the families we have studied, some people who inherit the gene develop the condition and others live their entire lives without showing any symptoms. There is a lot of research on genes and the environment that is attempting to understand how all these factors interact.
Genetic Testing in Parkinson’s Disease
Genetic testing has recently become available for the parkin and PINK1 genes. Parkin is a large gene and testing is difficult. At the current stage of understanding, testing is likely to give a meaningful result only for people who develop the condition before the age of 30 years. PINK1 appears to be a rare cause of inherited Parkinson’s disease. A small percentage of those developing the condition at an early age appear to carry mutations in the PINK1 gene. Genetic testing for the PARK7, SNCA and LRRK2 genes is also available.
Additional Resources
Also Check: Can Parkinson’s Run In The Family
Will Findings From Pd Ipsc Models Translate To Human Clinical Trials
One of the most exciting applications of patient-derived iPSC models of PD is to validate pharmacological treatments before clinical trials. The field is still at the stage of improving human brain tissue engineering, and many different protocols are being tested and developed. However, the need for progress in clinical translation for brain disorders is extremely high, and there is no time to wait for brain tissue models to be perfect. Pioneering iPSC studies pave the road to success and identify limitations which help the community to reach a consensus on the minimal requirements to model brain disorders in vitro most accurately. It seems essential to improve the efficiency of reprogramming and differentiation protocols while trying to make those models as physiological and realistic as possible,. Some concerns are raised that in vitro neuronal development, maturation and function might be too artificial, suggesting that the model may overlook some of the critical processes that occur in vivo. Nevertheless, some defects observed in iPSC-derived neurons have already been confirmed in human postmortem brain tissues,,,,. Although this is very encouraging, it is unclear whether significant in vitro phenotypes that cannot be confirmed in postmortem brain tissue should be disregarded. Most postmortem brains also have technical limitations and may represent later stages of the disease, whereas iPSC models may represent earlier stages, preceding neurodegeneration.
Full Financial Disclosures For The Previous 12months
O.A.A. received speaker honorarium from Lundbeck and is consultant for Healthlytix. A.M.D. reports that he was a founder of and holds equity in CorTechs Labs Inc. and serves on its scientific advisory board. He is a member of the scientific advisory board of Human Longevity, Inc., and the Mohn Medical Imaging and Visualization Centre. He received funding through research grants from GE Healthcare to University of California San Diego. The terms of these arrangements have been reviewed and approved by UCSD in accordance with its conflict-of-interest policies. T.M.S. reports honoraria, outside of the present work, from the University of Rochester, Varian Medical Systems, Multimodal Imaging Services Corporation, and WebMD. Z.G.-O. reports personal fees from Idorsia, Neuron23, Handl Therapeutics, Lysosomal Therapeutics Inc., Deerfield, Lighthouse, Prevail Therapeutics, Ono Therapeutics, Denali, and Inception Sciences outside the submitted work. D.G.G. has received honoraria for advisory board meetings from AbbVie and Bial Pharma, speaker fees from Britannia Pharmaceuticals and Bial Pharma, and consultancy fees from the Glasgow Memory Clinic. Other authors have nothing to report. L.P. has served as a consultant for Roche, for which a honorarium was paid to his employer, Oslo University Hospital.
Don’t Miss: Can Parkinson’s Run In The Family
Genetic Risk For Parkinson’s Disease
If you have a genetic mutation associated with Parkinson’s, will you get the disease? Not necessarily. Some mutations carry a greater risk, but none bring a 100 percent chance of developing Parkinson’s disease. There are many Parkinson’s risk genes where a mutation means a very small increased likelihood of Parkinson’s. Researchers are looking for other factors that either push or protect someone with a gene mutation to or from having Parkinson’s. Your doctor and/or a genetic counselor can discuss the risk associated with different Parkinson’s genes and what your results may mean for you and your loved ones.
New Genes For Recessive And X
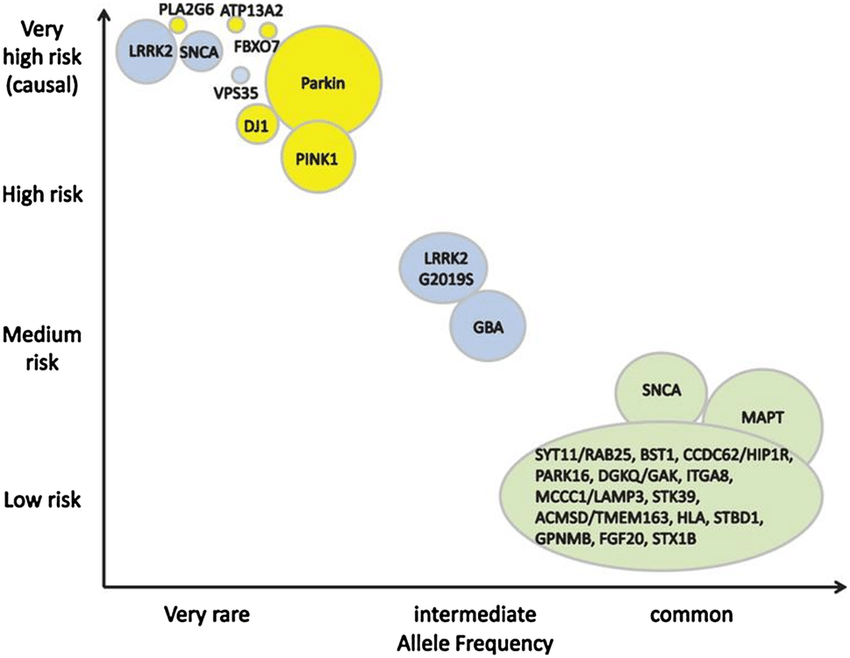
Compared to monogenic dominant PD and to the well-established recessive early-onset PD genes PARK2, DJ-1, and PINK1, the newly identified recessive forms appear more complex both for clinicians and researchers. The clinical picture of the newly identified recessive forms is often more severe and multifaceted. However, in a few instances, there appears to be a genotype-phenotype correlation where mutations that lead to pronounced alteration of normal protein function cause a complex disorder with severe additional neurological or neuropsychiatric impairment, often from birth, and juvenile Parkinsonism, whereas mutations with a milder effect on the protein cause Parkinsonism with fewer atypical features. Table 2 summarizes currently known and putative genes for recessive and X-linked PD or Parkinsonism.
Table 2 Monogenic causes for autosomal recessive or X-linked Parkinsons disease or atypical juvenile Parkinsonism
Recommended Reading: Can Parkinson’s Run In The Family
Evaluating The Potential For Model Improvement By Adding Snps Identified In Larger Gwas
An important disadvantage of the PHS approach is that the training step requires age data and individual genotypes, typically not available for data sets on the same scale as the largest GWAS meta-analyses. In a recent paper, we have shown that a PHS generated by our Cox regression method can be improved by the incorporation of GWAS-nominated SNPs in an additional step where the optimal SNP set is selected using least absolute shrinkage and selection operator -regularized regression. Following this published approach, we took advantage of results from the latest meta-analysis of GWAS in PD, where 83 SNPs were identified as genome-wide significant loci independently of our test data set in a âleave one outâ analysis. A list of SNPs combining our PHS model SNPs with these 83 GWAS SNPs was compiled, and the R package âglmnetâ was used to estimate a LASSO-regularized Cox proportional hazard model, where the hyper-parameter λ was selected using 10-fold cross-validation . A final LASSO model was estimated at the value of λ that minimized the mean cross-validated error, and the performance of the LASSO model was compared to the basic PHS approach described earlier.
Advancing Age And Parkinsons Disease
Age is perhaps the biggest risk factor for the onset of Parkinsons disease. The average age at which people will develop this movement disorder is 60. This is not usually something that affects younger people. The brain ages as people get older.
Even without external factors, cells in the substantia nigra can die on their own as an individual ages, causing symptoms to develop as the person gets older.
Don’t Miss: Adderall And Parkinson’s
Common Symptoms Of Parkinsons Disease
Here are some of the early signs and symptoms of Parkinsons disease that you need to be on the lookout for in deciding to see a healthcare professional who can provide medical advice.
- Rigid muscles
- Lack of ability to write
- Slowed movement
- Low blood pressure
If you notice these, or other symptoms involving the nervous system, it is time to see a doctor. If you experience a sudden drop in the bodys ability to execute any of these tasks or control movements, it is a sign that something is wrong. Doctors may be able to give medications or other treatments that could improve symptoms.
What Genes Are Linked To Parkinson’s Disease
In 1997, we studied a large family that came from a small town in Southern Italy in which PD was inherited from parent to child . We found the gene that caused their inherited Parkinson’s Disease and it coded for a protein called alpha-synuclein. If one studies the brains of people with PD after they die, one can see tiny little accumulations of protein called Lewy Bodies . Research has shown that there is a large amount of alpha-synuclein protein in the Lewy Bodies of people who have non-inherited PD as well as in the brains of people who have inherited PD. This immediately told us that alpha-synuclein played an important role in all forms of PD and we are still doing a lot of research to better understand this role.
Currently, seven genes that cause some form of Parkinson’s disease have been identified. Mutations in three known genes called SNCA , UCHL1 , and LRRK2 and another mapped gene have been reported in families with dominant inheritance. Mutations in three known genes, PARK2, PARK7 , and PINK1 have been found in affected individuals who had siblings with the condition but whose parents did not have Parkinson’s disease . There is some research to suggest that these genes are also involved in early-onset Parkinson’s disease or in dominantly inherited Parkinson’s disease but it is too early yet to be certain.
Also Check: What Color Represents Parkinson’s Disease
When To See A Doctor About Parkinsons
There isnt one specific test to diagnose Parkinsons disease. Doctors will usually evaluate your symptoms and perform several tests to determine if you have the condition. If you notice the following early warning signs, then you should see a doctor.
The early warning signs of Parkinsons disease include:
Causal And Associated Genes
The idea that a gene abnormality may cause some cases of Parkinsons dates back to 1997. At that time, scientists at the National Human Genome Research Institute and the National Institutes of Health first precisely identified that an irregularity in the synuclein alpha gene , the gene that provides instructions to make the protein alpha-synuclein, could lead to this movement disorder.
Alpha-synuclein is found in abundance in the brain and is thought to help regulate the release of dopamine, a chemical involved in the transmission of signals between nerve cells . With Parkinsons, the brain doesnt produce enough dopamine. This 1997 research on SNCA confirmed that at least one form of Parkinsons disease is inherited.
Up until 1997, people did not broadly think that Parkinsons could be hereditary or familial, says James Beck, PhD, chief scientific officer with the Parkinsons Foundation. With that discovery, we began to identify a number of genes linked with Parkinsons.
In 2004, scientists discovered the most common genetic contributor to Parkinsons, a mutation in LRRK2, a gene that is active in the brain and pushes a persons risk to 30 percent. Certain ethnic groups are more likely to have this gene irregularity. The faulty LRRK2 gene accounts for 1 percent to 2 percent of all Parkinsons cases, according to a review published in February 2016 in Biochemical Journal.
Read Also: Is Parkinson’s Disease Fatal
