Who Should Consider A Genetic Test For Parkinsons
There are two groups of people who might consider getting genetic testing and we will discuss each group separately.
Genetic testing for PD is a common request and a number of commercial labs perform panels of genetic testing for PD. You may ask: How can I test myself for Parksinons? Whether youre considering getting a genetic test through your doctor, or performing one at home, its important to note that at-home test dont map the entire gene for mutations. Genetic testing through your doctor will test for GBA, PARK7, SNCA, LRRK2, parkin and PINK1.
Both groups are faced with two questions: Should I get genetic testing? And if so, what should I do with the results? Before we address these two questions, we need to learn more about the complexity of genetic testing in PD.
Assessment Of Family History
The majority of our identified studies use self reporting or self administered questionnaires to assess family history of PD. Categorising PD cases who have relatives with isolated tremor as having a positive family history, can significantly increase the number of familial cases, especially among early onset PD cases.
Performing individual examinations may increase the precision with which a diagnosis of PD is made in relatives of cases and controls, rather than reliance on patient reporting of diagnoses or symptoms such as tremor. It has also been shown that significant numbers of previously unrecognised PD patients can be identified by examination despite a negative family history. It can often however be difficult verifying familial diagnoses in diseases affecting the elderly as relatives are often deceased and not subjected to postmortem examination. Subclinical Parkinson’s disease, diagnosed on the basis of Lewy body pathology in people without prior symptoms of PD, is observed in up to 10% of individuals subjected to postmortem neuropathological examination. No study includes pathological examination of all relatives of both cases and controls, which currently represents the gold standard in diagnosing PD.
Polymorphism Of Cyp2d6 Gene And Pesticide Exposure
The gene is primarily expressed in the liver and is responsible for the enzyme cytochrome P450 2D6. A study showed that those who had a mutation of this gene and were exposed to pesticides were twice as likely to develop Parkinson’s Disease; those that had the mutation and were not exposed to pesticides were not found to be at an increased risk of developing PD; the pesticides only had a “modest effect” for those without the mutation of the gene.
Genetic Testing For Parkinsons Disease
Similar to other complex diseases, the reason a particular person develops Parkinsons disease is likely a combination of genetic makeup and environment. In most people, the genetic contribution to disease development may be due to a number of different genes and the interactions between them. For only a very small percentage of people with PD, about 10%, the disease can be attributed to a single abnormal gene. Figuring out the identity and contributions of all the different genes that play a role in disease development is a very hot topic in PD research today.
What Is Lrrk2 Parkinson’s Disease
LRRK2 is a critical protein that regulates cellular function and is produced from theLRRK2 gene. G2019S LRRK2 Parkinsonâs disease is believed to be caused by a mutation in the LRRK2 gene leading to the production of overactive LRRK2. Most people with a diagnosis of Parkinson’s disease have not been tested for LRRK2 mutations, however it is estimated that between 3% -30% of patients have this underlying mutation. ESCAPE Bio is developing a G2019S LRRK2 targeted treatment.
Why Genetic Testing For Parkinsons Disease Is Complex:
- There are many genes that are associated with the development of PD. This list continues to grow as more genes are discovered. Testing of only some of these genes is available in commercial labs.
- The majority of people with PD, even those with a family history of PD, do not harbor one of these identified abnormal genes. The genetic contribution to PD in these people is yet to be discovered.
- For a particular gene there may be a number of different mutations associated with disease, some of which are more common than others. Commercial testing may identify only the most common of the mutations, and therefore not capture everyone who carries a disease-causing mutation.
- Conversely, only particular mutations in a gene may be associated with disease. Commercial testing may identify changes in a gene that may not have clinical consequences. This can be confusing for patients who even after genetic testing may not know whether they harbor a disease-causing mutation.
- Different mutations can be enriched in different ethnic populations. For example, Ashkenazi Jews and North African Berbers have an increased risk of carrying Leucine rich repeat kinase 2 mutations. Glucocerebrosidase mutation frequency also varies greatly with ethnicity and is also increased among Ashkenazi Jews.
In addition to the above, it is important to realize that not all genes associated with PD contribute to disease in the same way:
Human Ipsc Studies Of Pd Highlight Converging Molecular And Cellular Pathways Across Genetic Subgroups
Our analysis of 385 iPSC-derived cell lines from 67 published studies reveals that many PD neuronal phenotypes are shared between genetically heterogeneous familial and sporadic patients . Notably, impairments in mechanisms involved in cellular waste recycling, mitochondrial function, neuronal morphology and physiology, and sensitivity to reactive oxygen species are most common across patient lines with varying genetic predispositions . The studies measured cellular phenotypes that occurred either spontaneously or in response to chemicals mimicking cellular aging and stress . It is important to note that the frequency of reported phenotypes in our meta-analysis may be biased because only few studies reported negative results ,,,,,,,,,,,,. In addition, most cell lines were not systematically phenotyped without prior hypothesis and thus, there is likely to be an ascertain bias in these phenotypes. Less hypothesis-driven multimodal or omics analysis will help to address such bias,,,,,,,,. Phenotypes caused by genomic predispositions allude to crosstalk and impairments in multiple pathways that act collectively to mediate selective degeneration of dopaminergic neurons in the substantia nigra and will be discussed in detail below.
Fig. 4: Phenotypic insights from iPSC studies of Parkinsons disease.
Learning From Genetic Analyses Of Pd Casecontrol Studies
We analyzed the reports from 12 international studies,,,,,,,,,,,, totaling 5650 persons living with PD in North America, Europe, and Australia. We confirmed that globally only 15% of patients report a family history of PD symptoms, while the remaining 85% of the PD population are classified as sporadic PD . However, the distinction between genetic predispositions in familial and sporadic PD is blurry. No single-gene mutation in PD has a 100% penetrance. Instead, most likely, multiple genetic risk factors act in synergy to increase the chances of both familial and sporadic PD. Such genetic susceptibilities interplay with aging and environmental factors in both familial and sporadic PD.
Fig. 2: The genomics of Parkinsons disease: prevalence and penetrance.
a In the world-wide population of people living with PD, ~85% of PD cases are sporadic and the remaining are familial . b Genetic mutations occur at low and varying frequencies in the PD world population . Data represented as the mean±SEM. c GWAS data suggests risk variants in fPD genes tend to be less prevalent in PD cases . d Single nucleotide polymorphisms in over 44 genomic regions show significant association to PD. Each point presents an independent SNP hit associated with PD.
Genetic Risk For Parkinson’s Disease
If you have a genetic mutation associated with Parkinson’s, will you get the disease? Not necessarily. Some mutations carry a greater risk, but none bring a 100 percent chance of developing Parkinson’s disease. There are many Parkinson’s risk genes where a mutation means a very small increased likelihood of Parkinson’s. Researchers are looking for other factors that either push or protect someone with a gene mutation to or from having Parkinson’s. Your doctor and/or a genetic counselor can discuss the risk associated with different Parkinson’s genes and what your results may mean for you and your loved ones.
Advancing Age Is The Leading Risk Factor For Pd But Genetics Brain Injury And Exposure To Toxins May Play A Role Too
Parkinsons disease is a neurodegenerative disorder that affects approximately one million people in the U.S., and, to date, there is no cure for it. While effective treatments do exist to help you manage a wide array of PD symptoms, they cannot slow the progression of disease. And its not yet known whether, or how, the disease can be prevented. To solve that mystery, scientists first will have to unravel another riddle: what causes PD and which risks can up your odds for this condition to develop later on. Sharing leading-edge research and expert knowledge, well tell you all we know.
Genetic Mutation Linked To Parkinson’s Disease
- Date:
- Mayo Clinic
- Summary:
- Researchers have discovered a new gene mutation they say causes Parkinson’s disease. The mutation was identified in a large Swiss family with Parkinson’s disease, using advanced DNA sequencing technology.
Researchers have discovered a new gene mutation they say causes Parkinson’s disease. The mutation was identified in a large Swiss family with Parkinson’s disease, using advanced DNA sequencing technology.
The study, published July 15 in the American Journal of Human Genetics, was led by neuroscientists at the Mayo Clinic campus in Florida and included collaborators from the U.S., Canada, Europe, United Kingdom, Asia and the Middle East.
“This finding provides an exciting new direction for Parkinson’s disease research,” says co-author Zbigniew Wszolek, M.D., a Mayo Clinic neuroscientist. “Every new gene we discover for Parkinson’s disease opens up new ways to understand this complex disease, as well as potential ways of clinically managing it.”
“There is much more we need to know about this gene,” Dr. Ross says. “Although it appears to be a rare cause of Parkinson’s disease, it seems to be very important from a mechanistic viewpoint for this disease and possibly other neurodegenerative disorders.”
Story Source:
Key Gene Mutations Associated With Parkinson’s
There are forms of Parkinson’s that appear to be influenced by genetic defects that run in families. We tend to see this with early-onset forms of the disease wherein symptoms being to appear far earlier than average onset age of 60.
One type of genetic mutation associated with familial parkinsonism is in the so-called SNCA gene. This is the gene linked with the production of alpha-synuclein protein, a biomolecule which can contribute to abnormalities in nerve cells. While rare in the general population, the SNCA gene mutation has been identified in around two percent of families affected by Parkinson’s.
In 2004, scientists discovered a similar genetic mutation in a number of families in whom multiple members had been affected. The so-called LRRK2 mutation is today linked to about one to two percent of all Parkinson cases, mostly affecting people of Jewish, Ashkenazi, North African Arab-Berber, or Basque origin.
Another mutation involving the GBA gene is already known to cause Gaucher’s disease . Research has since shown that the GBA mutation is present in a significant number of people with Parkinson’s, suggesting a causal link between the mutation and the disease.
What Are The Symptoms
Parkinsons patients often have trouble walking and talking. Symptoms include slowness of movement, a loss of balance and slurred speech. With Parkinsons disease, you may have a decreased ability to perform unconscious movements, including blinking, smiling or swinging your arms when you walk, the Mayo Clinic says. The symptoms are often worse on one side of the body.
Genetic Susceptibility Factors In Parkinson’s Disease
Monogenic forms represent less than 10% of PD in most populations. The vast majority result from complex interactions among genes and between genes and environmental factors. Genetic variations may be susceptibility factors or disease modifiers, affecting penetrance, age at onset, severity and progression. High-density arrays of single nucleotide polymorphisms permit the identification of susceptibility factors in genome-wide association studies, in which the frequencies of putative risk alleles are compared in patients and controls.
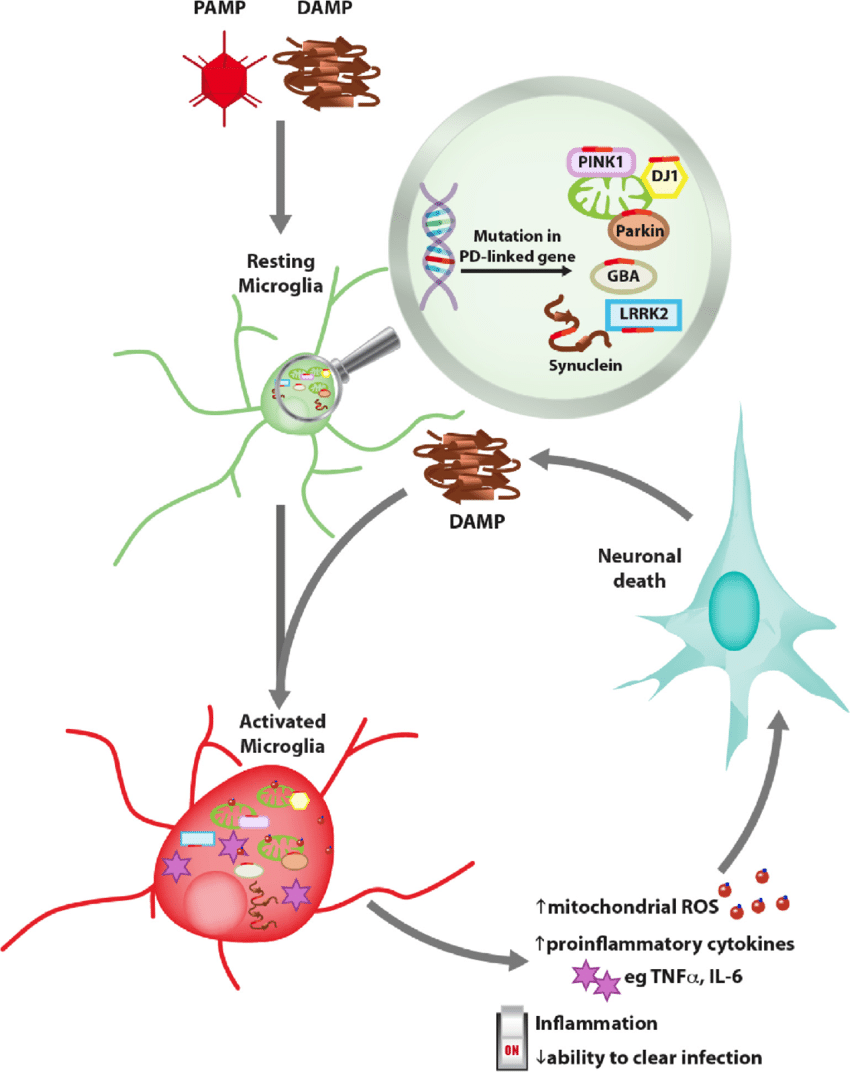
Neuroinflammation Exacerbates Neurodegeneration In Sporadic Pd
Midbrain neurons derived from sporadic patients showed increased susceptibility to the effects of adaptive immune cells. Sporadic patient neuronal lines co-cultured with T-lymphocytes exhibited substantial signs of cell death mediated by IL-17IL-17R signaling and activation of NFkB. Similarly, IL-17 treatment resulted in increased neuronal death. Inflammation in the central nervous system and periphery are key hallmarks of PD. Increasing evidence implicates the role of microglia in neuronal loss, though the underlying mechanisms remain to be determined,. RNA-seq analysis of astrocytes derived from LRRK2-G2019S iPSCs highlighted dysregulation in genes involved in the extracellular matrix, which may reduce the neuroprotective capacity of astrocytes in PD. Investigating the role of neuroinflammation in patient-derived microglia may also contribute to the understanding of the selective vulnerability of mDA neurons in sporadic and late-onset PD.
What Is Parkinsons Disease
This slow-moving disease harms nerve cells in your brain over time, causing them to slowly die. Most of those affected nerves control and coordinate your bodys movements, triggering Parkinsons disease motor symptoms. As PD progresses, it becomes harder to control muscle movement and coordination. So getting dressed, walking, and other daily activitiesthings that used to be easycan become more and more challenging.
Diana Teixeira And Ins Lopes Cardoso*
*Corresponding author:Received:Accepted:KeywordsParkin
Cite this as
Background:Parkinsons disease is the second most important neurodegenerative disorder, affecting 3% of individuals older than 80 years of age. Main clinical symptoms are resting tremor, postural instability, bradykinesia and rigidity, with a good response to levodopa therapy.
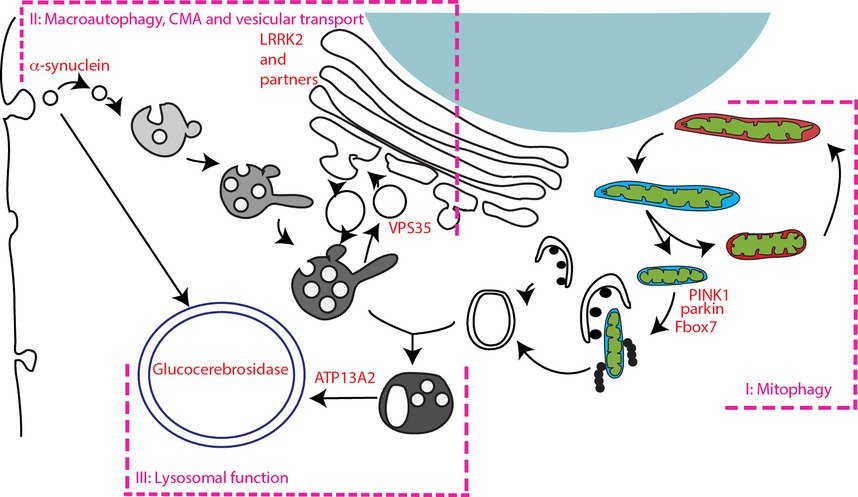
Purpose of the study: The main goal of this work is to make a deep analysis on the genetic factors and kind of heritage involved in the development of Parkinson.
Main findings: Over the last years, numerous studies allowed to conrm the unquestionable contribution of genetic factors to the complex pathogenesis of this disease. Highly penetrant mutations producing rare, monogenic forms of the disease have been identified in singular genes such as SNCA, Parkin, DJ-1, PINK1, LRRK2, and VPS35. Unique variants with incomplete penetrance in LRRK2 and GBA genes were identified as strong risk factors for Parkinsons disease in certain populations. Additionally, over 20 common variants with small effect sizes are now recognized to modulate the risk for Parkinsons disease.
Investigating Mendelian forms of Parkinson disease has provided precious insight into the pathophysiology that underlies the more common idiopathic form of this disorder.
Main article text
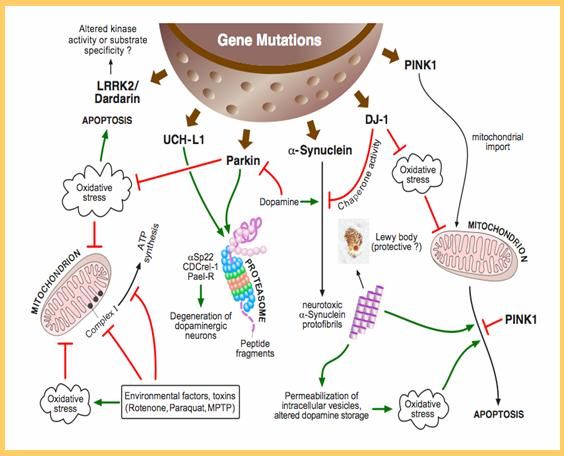
Insights Into Mutations That Cause Parkinsons Disease
Researchers determined how an abnormal gene begins the process that leads to neuron death and Parkinsons disease. The finding hints at potential new therapies for the movement disorder.
Parkinsons disease is a degenerative disorder that destroys neurons in the brain. The loss of neurons leads to movement difficulties that include trembling of the hands, arms, legs, jaw, or head; slowed movements; muscle stiffness; and impaired balance. Medications or surgery can often improve symptoms, but currently theres no cure.
While the exact causes of Parkinsons disease arent known, scientists have identified several genes that are linked to the disorder. Misspellings in one gene, LRRK2 are the most common genetic cause of the disease. LRRK2 mutations have been implicated in about 10% of inherited forms of Parkinsons and in about 4% of patients with no family history of the disease. The most common LRRK2 mutation, called G2019S, is thought to be the cause of 30-40% of Parkinsons cases in people of North African Arabic descent.
A team led by Drs. Ted Dawson and Valina Dawson at the Johns Hopkins University set out to learn why mutations in the LRRK2 gene might lead to Parkinsons disease. Their study was funded in part by NIHs National Institute of Neurological Disorders and Stroke . Results appeared online on April 10, 2014, in Cell.
These findings suggest that misspellings in the LRRK2 gene may lead to Parkinsons disease by abnormally increasing protein synthesis in neurons.
New Finding On Parkinsons Gene Mutation Alters View Of What Causes The Disease
Scientists report that the most common Parkinson’s gene mutation may change how immune cells react to generic infections like colds, which in turn trigger the inflammatory reaction in the brain that causes Parkinson’s. Their study , published in Brain, contradicts the long-held view that Parkinson’s was a disease that starts in the brain, destroying motion centers and resulting in the tremors and loss of movement.
Missense mutations in the leucine rich repeat kinase 2 gene result in late-onset Parkinsons disease. The incomplete penetrance of LRRK2 mutations in humans and LRRK2 murine models of Parkinsons disease suggests that the disease may result from a complex interplay of genetic predispositions and persistent exogenous insults. Since neuroinflammation is commonly associated with the pathogenesis of Parkinsons disease, we examine a potential role of mutant LRRK2 in regulation of the immune response and inflammatory signaling in vivo. Here, we show that mice overexpressing human pathogenic LRRK2 mutations, but not wild-type mice or mice overexpressing human wild-type LRRK2 exhibit long-term lipopolysaccharide-induced nigral neuronal loss. This neurodegeneration is accompanied by an exacerbated neuroinflammation in the brain, write the investigators.
New Genes For Dominant Pd
Since 2012, mutations in DNAJC13, CHCHD2, TMEM230, and RIC3 have been reported as new causes for monogenic dominant PD. The following sections provide a review of the available reports on the clinical picture and genetic information. All of these discoveries are recent, and it is today not definitely proven that mutations in these genes cause PD. The present evidence appears most robust for a causative role of CHCHD2 mutations in PD because of the description of more than one family with co-segregation of clinical phenotype and genotype, but less robust for DNAJC13 and TMEM230, because these mutations were found in only one, albeit large, pedigree, and least robust for RIC3 that has only been reported from one less extensive family. Table provides an overview over presently known genes for dominant PD, which generally cause medium- to late-onset Parkinsonism or PD, for most genes with few or no additional features.
Table 1 Monogenic causes for autosomal dominant Parkinsons diseaseFull size table
Genetic Role Not Entirely Known In Affected Families
editorial processSarah Rahal, MDMedical Review Board
Genetics very likely plays a role in all types of Parkinson’s disease. However, while having a specific combination of genetics may increase your risk of the disease, it doesn’t necessarily mean that you’ll get it.
Around 15 to 25 percent of people living with Parkinson’s have a family history of the condition, either an immediate or second-degree relation. Having one or more of these relatives will place you at slightly higher risk for Parkinson’s, but it’s still no guarantee you’ll develop the disorder.
Conversely, if you have Parkinson’s, it shouldn’t suggest that any of your kids or grandkids will get the disease either. It merely indicates that their risk is slightly above those without a family history.
In the end, most cases of Parkinson’s don’t have any known cause . While there are forms that seem to run in families, these account for a small percentage of cases roughly five to 10 percent, all told.
Identification Of New Genes And Risk Factors For Pd
New PD-linked genes or PD risk factors can be identified by gene mapping or candidate gene approaches. Gene mapping in human diseases is the localization of genes underlying the clinical phenotypes of the disease on the basis of correlation with DNA variants , without the need for prior hypotheses about biological function. Genetic mapping methods include linkage analysis and genome-wide association studies. Alternatively, based on their known function, levels of expression, or mode of interaction , some genes can be considered plausible candidates, and as such, tested for in cohorts of patients.
Omics Analysis Of Patient
To date 10/67 iPSC-PD studies analyzed have used proteomic, transcriptomic, or epigenomic profiling to phenotype PD patient-derived neurons,,,,,,,,. Omics analyses may be less biased and data-driven as opposed to purely hypothesis-driven. Data from omics studies can also help to describe biological relationships between complex intertwined cellular pathways and identify relevant druggable molecular pathways.
New Genes For Recessive And X
Compared to monogenic dominant PD and to the well-established recessive early-onset PD genes PARK2, DJ-1, and PINK1, the newly identified recessive forms appear more complex both for clinicians and researchers. The clinical picture of the newly identified recessive forms is often more severe and multifaceted. However, in a few instances, there appears to be a genotype-phenotype correlation where mutations that lead to pronounced alteration of normal protein function cause a complex disorder with severe additional neurological or neuropsychiatric impairment, often from birth, and juvenile Parkinsonism, whereas mutations with a milder effect on the protein cause Parkinsonism with fewer atypical features. Table summarizes currently known and putative genes for recessive and X-linked PD or Parkinsonism.
Table 2 Monogenic causes for autosomal recessive or X-linked Parkinsons disease or atypical juvenile ParkinsonismFull size table
Are You At Higher Risk
It is relatively rare for a parent to pass down Parkinsons to a child. However, people who get early-onset Parkinsons are more likely to have inherited it.
In most cases, the cause of Parkinsons is unknown, although the most common risk factors include:
- Age
- Male gender
- Mutations in specific genes associated with Parkinsons
- A family history of Parkinsons
- Exposure to herbicides or pesticides
The only way to truly determine if you or a member of your family is carrying one of the genes associated with the disease is to participate in genetic testing. These tests can identify if you have an associated gene marker and how likely it is that it will be passed down to your children.
While there isnt a way to fully prevent Parkinsons disease, you can certainly be proactive, Dr. Hanna says. Genetic testing and counseling will allow you to discover if you do have a mutated gene, how likely it is that it will be passed down to your children, as well as the best treatment options for your diagnosis.
Genetic Classification Of Pd
In the current PD genetics nomenclature, 18 specific chromosomal regions, also called chromosomal locus, are termed PARK , and numbered in chronological order of their identification . In addition to being an incomplete list of known PD-related genes, this classification system, unfortunately, has a number of inconsistencies. It comprises confirmed loci, as well as those for which linkage or association could not be replicated . The causative gene has not yet been identified for all of the loci, nor do all of the identified genes contain causative or disease-determining mutations . Finally, one locus, PARK4, was designated as a novel chromosomal region associated with PD but was later found to be identical with PARK1 . It is noteworthy that some of the loci have been identified by genetic linkage analysis in large families, some based on the known function of the protein product of the gene they contain, yet others have been established by genome-wide association studies performed on a population level. A list of the PARK PD-related genes and loci is given in , along with their clinical classification, inheritance pattern , gene , status , and mode of identification.
Can Parkinsons Be Passed From Parent To Child
Its rare for Parkinsons disease to be passed down from parent to child. Most cases of Parkinsons arent hereditary. But people who get early-onset Parkinsons disease are more likely to have inherited it.
Having a family history of Parkinsons disease may increase the risk that youll get it. This means that having a parent or sibling with Parkinsons slightly increases the risk.
In most cases, the cause of Parkinsons disease remains unknown. But researchers have identified multiple risk factors that can increase your chances of getting this disease.
Risk factors for Parkinsons disease include:
- mutations in specific genes associated with Parkinsons
- having a family history of Parkinsons or a first-degree family member with Parkinsons
- being older, especially above the age of 60
- exposure to herbicides and pesticides
- being assigned male at birth
- history of brain injury
