Human Ipsc Studies Of Pd Highlight Converging Molecular And Cellular Pathways Across Genetic Subgroups
Our analysis of 385 iPSC-derived cell lines from 67 published studies reveals that many PD neuronal phenotypes are shared between genetically heterogeneous familial and sporadic patients . Notably, impairments in mechanisms involved in cellular waste recycling, mitochondrial function, neuronal morphology and physiology, and sensitivity to reactive oxygen species are most common across patient lines with varying genetic predispositions . The studies measured cellular phenotypes that occurred either spontaneously or in response to chemicals mimicking cellular aging and stress . It is important to note that the frequency of reported phenotypes in our meta-analysis may be biased because only few studies reported negative results 31,32,36,37,40,45,48,52,59,64,74,76,86. In addition, most cell lines were not systematically phenotyped without prior hypothesis and thus, there is likely to be an ascertain bias in these phenotypes. Less hypothesis-driven multimodal or omics analysis will help to address such bias41,72,76,77,78,79,80,87,90. Phenotypes caused by genomic predispositions allude to crosstalk and impairments in multiple pathways that act collectively to mediate selective degeneration of dopaminergic neurons in the substantia nigra and will be discussed in detail below.
Fig. 4: Phenotypic insights from iPSC studies of Parkinson’s disease.
The Interplay Between Genomic Predispositions And Environmental Factors Leads To Parkinsons
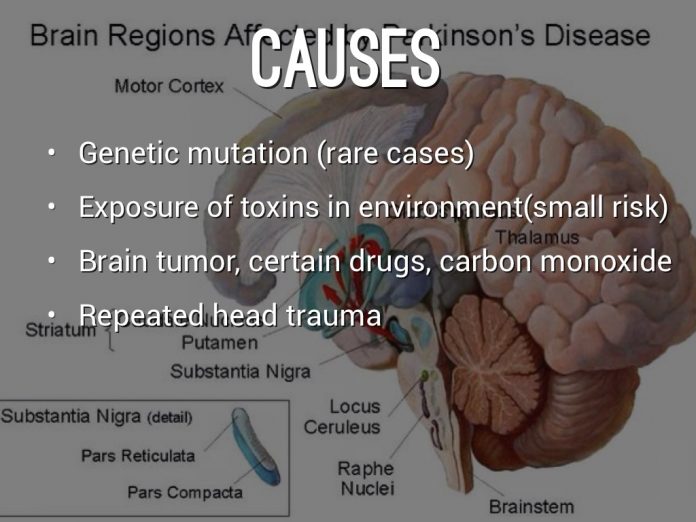
In the mid-1990s, the connection between PD and underlying genetic mutations was established4,5,150. It is now evident that varying degrees of the interplay between genomic predispositions and aging and cellular stressors impose a risk for disease151 . Previous studies have shown vascular insults to the brain, repeated head trauma, neuroleptic drugs, exposure to pesticides, and manganese toxicity increase the risks of developing symptoms of PD152,153,154. In addition, advancing age can also cause a cascade of stressors within the substantia nigra, which weakens the neurons and their ability to respond to further insults155,156. Ultimately, the uniqueness of the interactions between genes and the environment makes the development of a single treatment for PD difficult as they give rise to a spectrum of neuronal phenotypes that can be unique to individual patients . The development of a model with the ability to replicate the genomic and epigenetic aspects of the disease is crucial . As increasing evidence suggests that genetic mutations are key modulators of disease initiation and progression, the identification and understanding of the various genomic predispositions are required for the development of better-targeted treatments to slow the disease progression.
Fig. 1: A combinatorial spectrum of genetic risks, cellular stressors, and brain cell dysfunctions causes Parkinson’s disease.Full size image
What Is The Difference Between Progressive Supranuclear Palsy & Parkinsons Disease
Both PSP and Parkinson’s disease show similar symptoms such as stiffness, movement difficulties, and clumsiness, but the severity of the condition is often based on the symptoms.1
Progressive supranuclear palsy is more progressive when compared to Parkinson’s disease.2
PSP is a rare brain disorder that causes serious and progressive problems whereas Parkinson’s disease is a nervous system disorder that affects movements.3,4
Many studies were conducted based on the facts of the two diseases that presented very similar symptoms, but they are quite different from each other when analyzed in detail.
PSP and Parkinson’s have overlapping symptoms, but diagnosis often becomes complicated. However, experts suggest that PSP is more severe with cognitive impairment when compared to Parkinson’s disease. Concentration and memory are most severely affected in patients with progressive supranuclear palsy.
Genetic Predispositions Reducing Differentiation Yield Of Mda Neurons
In vitro neural development was impaired in neural lines derived from patients carrying LRRK2, PRKN, SNCA, and sporadic mutations43,49,74,93. In four independent studies, the differentiation potential of neural progenitor cells derived from patients was significantly reduced, demonstrated by low yields of neurons in comparison with control lines43,49,74,81,94. A recent review presented the idea that PD is attributed to significant neurodevelopmental defects, which may increase the susceptibility for disease onset224. If confirmed, identifying genetic predispositions that contribute to early developmental defects in iPSC-PD may assist the development of novel PD therapies. However, these phenotypes may appear in conflict with other studies53,55,76 capable of generating functional neurons from cell lines with similar mutations. The differences could be due to varying protocols, which may be more or less stressful for the cells.
Will Findings From Pd Ipsc Models Translate To Human Clinical Trials
Given the apprehensions that in vitro studies may be too artificial, human iPSC-derived neural progenitors may be transplanted into animal brains244,245,246,247. Besides ethical barriers, xenografts also raise the possibility that the healthy host tissue compensate for the impairment of the transplanted cells. Yet, if the phenotypes observed in vitro are recapitulated in vivo, pharmacological treatments could be assessed in a systemic environment, with much more realistic dosage and administration methods.
How Environmental Factors And Aging Can Be Recapitulated In Vitro
An obvious limitation of in vitro models is the lack of environmental context. The influence of nongenetic factors is not recapitulated in the basal phenotype of patient-derived neurons. For example, the influence of head trauma of a boxer with sporadic PD will not be recapitulated by default in reprogrammed neurons. An alternative would be to transplant the patient-derived neurons in animals and simulate the trauma on the animal. Similarly, influence of decades of aging of the human brain is difficult to reproduce in vitro in a few months within the boundaries of feasible experimental design. Brains in a dish will always be an imperfect experimental model. However, many tricks can be used to recapitulate the environmental and aging stress in vitro. Table 2 summarizes a list of reagents that have already been used in iPSC neuronal culture to mimic oxidative stress, proteostatic stress, mitochondrial stress, synaptic stress, ER stress, inflammation, and cellular aging. An interesting example is progerin, a truncated form of lamin A associated with premature aging. Increasing the expression of progerin in iPSC neurons can recapitulate at least some aspect of cellular aging in vitro71. Human iPSC-derived dopamine neurons overexpressing progerin displayed specific phenotypes such as neuromelanin accumulation. In addition, PD patient-derived neurons revealed disease-related phenotypes that required both genetic susceptibility and induced-aging in vitro71.
Projected Estimates Of Parkinsons Disease With Aging Population
As the life expectancy has increased worldwide, it is expected that the burden of chronic diseases, like PD, will continue to grow. It is estimated that the number of people with PD in 2005 totaled between 4.1 million and 4.6 million and that number will more than double by 2030 to between 8.7 million and 9.3 million.7
Neuroinflammation Exacerbates Neurodegeneration In Sporadic Pd
Midbrain neurons derived from sporadic patients showed increased susceptibility to the effects of adaptive immune cells72. Sporadic patient neuronal lines co-cultured with T-lymphocytes exhibited substantial signs of cell death mediated by IL-17–IL-17R signaling and activation of NFkB72. Similarly, IL-17 treatment resulted in increased neuronal death72. Inflammation in the central nervous system and periphery are key hallmarks of PD220. Increasing evidence implicates the role of microglia in neuronal loss, though the underlying mechanisms remain to be determined221,222. RNA-seq analysis of astrocytes derived from LRRK2-G2019S iPSCs highlighted dysregulation in genes involved in the extracellular matrix, which may reduce the neuroprotective capacity of astrocytes in PD78. Investigating the role of neuroinflammation in patient-derived microglia may also contribute to the understanding of the selective vulnerability of mDA neurons in sporadic and late-onset PD223.
Identification Of Rare Variants In Pd Loci And Candidate Genes

Exome sequencing on 109 individuals resulted in an overall average coverage >100×. The average PD onset age is 35.9 years across 85 patients. The average age at collection is 55.8 years for PD patients and 66.4 years across 24 controls. The percentage of male patients is 62% in the patient cohort and 38% in the control cohort. We did not have the power for association analysis when separating individuals with a FH and sporadic cases. No individuals were related to each other besides the one set of siblings reported in this study. After applying our standard filtering criteria of minor allele frequency < 0.01 in Genome Aggregation Database , functional impact: non-synonymous, stop-gain, frameshift, and splicing variants, CADD score > 15, and Phred quality score > 20, coverage > 20×, and variant allele fraction > 40% of called reads, we identified 54 unique variants in 71 candidate genes; of these variants, 49 were not observed in healthy controls . However, we acknowledge the limitation of the small sample size of our discovery cohort. Furthermore, 6 out of 49 variants were present in multiple PD cases and located in five genes , STAB1: c.3265A>G: p.S1089G , ANK2: c.10901T>A: p.V3634D , ANK2: c.11716C>T: p.R3906W , SH3GL2: c.827G>T: p.G276V , NOD2: c.2722G>C: p.G908R .
The Importance Of Establishing Parkinsons Prevalence Numbers
Parkinson’s Prevalence estimates will help the Parkinson’s Foundation attract the attention of federal and state government as well as the pharmaceutical industry to the growing need and urgency in addressing PD. This is an important first step to better understanding who develops PD and why.
The next phase of this study will be to determine the rate of PD diagnosis or incidence, how that has changed over time and what is the rate of mortality among those affected by PD. Determining the prevalence and incidence will allow the PD community to effectively advocate for additional money and resources necessary to support Parkinson’s research.
Parkinson’s Foundation Prevalence Project numbers highlight the growing importance of optimizing expert Parkinson’s care and treatment for people with Parkinson’s, which would help future caregivers and ease the strain on health and elder care systems.
Loading…
Mri Technique In Distinguishing Psp And Parkinsons Disease
A medical study was conducted on 10 patients who showed motor symptoms. All the 10 patients have Parkinson’s and 5 patients were experiencing vertical supranuclear gaze palsy with increased imaging biomarker values and slowness in vertical saccades.
MRI was performed on these patients that included both MRPI and MRPI 2.0. The values showed different ranges between these patients and it also helped to identify the condition during the early stage of the symptoms.
Clinical variables assessed the motor functions and cognitive functions. Results were prepared based on the imaging biomarkers and clinical variables and it showed that disease progression was most severe in PSP when compared to Parkinson’s disease.3,4
Also Read:
What Are The Symptoms Of Atypical Parkinsonian Disorders
Like classic Parkinson’s disease, atypical Parkinsonian disorders cause muscle stiffness, tremor, and problems with walking/balance and fine motor coordination.
Patients with atypical Parkinsonism often have some degree of difficulty speaking or swallowing, and drooling can be a problem. Psychiatric disturbances such as agitation, anxiety or depression may also be part of the clinical picture.
Dementia with Lewy bodies can cause changes in attention or alertness over hours or days, often with long periods of sleep during the day. Visual hallucinations — typically of small animals or children, or moving shadows in the periphery of the visual field — are common in DLB. DLB is second only to Alzheimer’s disease as a cause of dementia in the elderly, and it most commonly affects patients in their 60s.
Patients with progressive supranuclear palsy may have difficulties with eye movements, particularly when looking downward, and with balance — when descending stairs, for instance. Backward falls are common and may occur during the early course of the disease. PSP is not usually associated with tremor, unlike Parkinson’s disease.
Parkinson’s Disease and Movement Disorders Center
Learning From Genetic Analyses Of Pd Casecontrol Studies

We analyzed the reports from 12 international studies94,157,158,159,160,161,162,163,164,165,166,167, totaling 5650 persons living with PD in North America, Europe, and Australia. We confirmed that globally only 15% of patients report a family history of PD symptoms, while the remaining 85% of the PD population are classified as sporadic PD . However, the distinction between genetic predispositions in familial and sporadic PD is blurry. No single-gene mutation in PD has a 100% penetrance. Instead, most likely, multiple genetic risk factors act in synergy to increase the chances of both familial and sporadic PD. Such genetic susceptibilities interplay with aging and environmental factors in both familial and sporadic PD.
Fig. 2: The genomics of Parkinson’s disease: prevalence and penetrance.
a In the world-wide population of people living with PD, ~85% of PD cases are sporadic and the remaining are familial . b Genetic mutations occur at low and varying frequencies in the PD world population . Data represented as the mean±SEM. c GWAS data suggests risk variants in fPD genes tend to be less prevalent in PD cases . d Single nucleotide polymorphisms in over 44 genomic regions show significant association to PD. Each point presents an independent SNP hit associated with PD.
Estimated Healthcare Costs Related To Pd In The Us
The combined direct and indirect cost of Parkinson’s, including treatment, social security payments and lost income, is estimated to be nearly $52 billion per year in the United States alone.
Medications alone cost an average of $2,500 a year and therapeutic surgery can cost up to $100,000 per person.
Lewy Body Dementia Vs Parkinsons Disease Dementia
Diagnoses of Lewy body dementia include dementia with Lewy bodies and Parkinson’s disease dementia. Symptoms in both of these diagnoses can be similar.
Lewy body dementia is a progressive dementia caused by abnormal deposits of a protein called alpha-synuclein in the brain. Lewy bodies are also seen in Parkinson’s disease.
The overlap in symptoms between Lewy body dementia and Parkinson’s disease dementia include movement symptoms, rigid muscles, and problems with thinking and reasoning.
This seems to indicate that they could be linked to the same abnormalities, though more research is needed to confirm that.
The later stages of Parkinson’s disease have more severe symptoms that may require help moving around, around-the-clock care, or a wheelchair. Quality of life can decline rapidly.
Risks of infection, incontinence, pneumonia, falls, insomnia, and choking increase.
Hospice care, memory care, home health aides, social workers, and support counselors can be a help in later stages.
Parkinson’s disease itself isn’t fatal, but complications can be.
Research has shown a median survival rate of about
Epigenomic Alterations Linked With Pd In Patient
The ability to capture unique epigenomic alterations associated with PD remains an important challenge. Reprogramming fibroblasts to iPSCs may erase age-associated225 and naive epigenetic signatures which could contribute to sporadic PD pathophysiology226. However, an epigenetic phenotype was reported in iPSC-derived PD patient neurons79,89. Neuronal lines derived from LRRK2 and sporadic patients exhibited epigenomic alterations when compared with healthy controls79. Hypermethylation was prominent in gene regulatory regions associated with the downregulation of transcription factors FOXA1, NR3C1, HNF4A, and FOSL279. Interestingly, LRRK2 mutant and sporadic PD patient neurons shared similar methylation patterns, which were absent in the original donor fibroblasts79. A spontaneous increase in the number of DNA strand breaks and genomic damage89 in PD patient-derived neurons could indirectly impact genomic regulation.
Conditions Misdiagnosed As Parkinson’s Disease

Parkinsons disease, especially in its early stages when symptoms are mild, is not an easy disease to diagnose. The non-specific, and easily overlooked nature of the signs of Parkinsons make it difficult to spot, and unlike many illnesses, there is no one laboratory test or radiological exam that will provide a definitive diagnosis of Parkinsons disease.
Patients exhibiting Parkinsons-like symptoms may undergo blood and urine tests, or CT or MRI scans to exclude other conditions, but none of these will provide a diagnosis of Parkinsons disease. The best way to test for Parkinsons disease is to conduct a systemic neurological examination that includes tests to gauge a patients reflexes, muscle strength, coordination, balance, gait, and overall movement. Even so, according to information presented on The Michael J. Fox Foundation for Parkinsons Research, up to 25 percent of Parkinsons disease diagnoses are incorrect.
So, why is there confusion about diagnosing Parkinsons disease? The simple answer is that symptoms of Parkinsons disease are not clear cut, and therefore, it is easy to mistake them for other conditions, or to classify them as parkinsonian when they are not.
Here is a brief overview of the top ten conditions mistaken for Parkinsons disease:
Beyond those top three, there are other conditions that are often confused with Parkinsons disease, including:
Generating Relevant Neuronal Cell Types For Pd
The cellular reprogramming toolbox for researchers is rapidly expanding and includes a panoply of neuronal differentiation protocols to generate cells representing various brain regions21. PD is a debilitating motor system disorder resulting from the selective degeneration of midbrain dopamine neurons located in the substantia nigra pars compacta. Protocols have been established to specifically generate dopaminergic neurons and brain cells with a midbrain molecular profile195,196.
Can Parkinsons Be Passed From Parent To Child
It’s rare for Parkinson’s disease to be passed down from parent to child. Most cases of Parkinson’s aren’t hereditary. But people who get early-onset Parkinson’s disease are more likely to have inherited it.
Having a family history of Parkinson’s disease may increase the risk that you’ll get it. This means that having a parent or sibling with Parkinson’s slightly increases the risk.
In most cases, the cause of Parkinson’s disease remains unknown. But researchers have identified multiple risk factors that can increase your chances of getting this disease.
Risk factors for Parkinson’s disease include:
- mutations in specific genes associated with Parkinson’s
- having a family history of Parkinson’s or a first-degree family member with Parkinson’s
- being older, especially above the age of 60
- exposure to herbicides and pesticides
- being assigned male at birth
- history of brain injury
How Is Psp Different From Parkinson’s Disease
PSP is often misdiagnosed as Parkinson’s disease, especially early in the disorder, as they share many symptoms, including stiffness, movement difficulties, clumsiness, bradykinesia , and rigidity of muscles. The onset of both diseases is in late middle age. However, PSP progresses more rapidly than Parkinson’s disease.
- People with PSP usually stand exceptionally straight or occasionally tilt their heads backward . This is termed “axial rigidity.” Those with Parkinson’s disease usually bend forward.
- Problems with speech and swallowing are much more common and severe in PSP than in Parkinson’s disease and tend to show up earlier in the disease.
- Eye movements are abnormal in PSP but close to normal in Parkinson’s disease.
- Tremor is rare in PSP but very common in individuals with Parkinson’s disease.
Although individuals with Parkinson’s disease markedly benefit from the drug levodopa, people with PSP respond minimally and only briefly to this drug.
People with PSP show accumulation of the protein tau in affected brain cells, whereas people with Parkinson’s disease show accumulation of a different protein called alpha-synuclein.
Behaviors Seen In Parkinsons Disease Dementia
As dementia progresses, managing disorientation, confusion, agitation, and impulsivity can be a key component of care.
Some patients experience hallucinations or delusions as a complication of Parkinson’s disease. These may be frightening and debilitating. Approximately 50 percent of those with the disease may experience them.
The best thing to do when giving care to someone experiencing hallucinations or delusions from Parkinson’s disease dementia is to keep them calm and reduce their stress.
Take note of their symptoms and what they were doing before they exhibited signs of hallucinating and then let their doctor know.
This element of the disease can be particularly challenging for caregivers. Patients may become unable to care for themselves or be left alone.
Some ways to make caregiving easier include:
- sticking to a normal routine whenever possible
- being extra comforting after any medical procedures
- limiting distractions
- using curtains, nightlights, and clocks to help stick to a regular sleep schedule
- remembering that the behaviors are a factor of the disease and not the person
How Is Parkinsons Disease Dementia Diagnosed
No single test can diagnose Parkinson’s disease dementia. Instead, doctors rely on a series or combination of tests and indicators.
Your neurologist will likely diagnose you with Parkinson’s and then track your progression. They may monitor you for signs of dementia. As you get older, your risk for Parkinson’s dementia increases.
Your doctor is more likely to conduct regular testing to monitor your cognitive functions, memory recall, and mental health.
What Are The Different Forms Of Parkinsonism
There are three main forms of parkinsonism, as well as other related conditions.
Most people with parkinsonism have idiopathic Parkinson’s disease, also known as Parkinson’s. Idiopathic means the cause is unknown.
The most common symptoms of idiopathic Parkinson’s are tremor, rigidity and slowness of movement.
Vascular parkinsonism affects people with restricted blood supply to the brain. Sometimes people who have had a mild stroke may develop this form of parkinsonism.
Common symptoms include problems with memory, sleep, mood and movement.
Some drugs can cause parkinsonism.
Neuroleptic drugs , which block the action of the chemical dopamine in the brain, are thought to be the biggest cause of drug-induced parkinsonism.
The symptoms of drug-induced parkinsonism tend to stay the same – only in rare cases do they progress in the way that Parkinson’s symptoms do.
Drug-induced parkinsonism only affects a small number of people, and most will recover within months – and often within days or weeks – of stopping the drug that’s causing it.
Rare Variants In Pathogenic Variant Carriers
Although this is an underpowered assessment of double mutations with only 23 patients, we thought that a descriptive and exploratory analysis is still warranted for pathogenic mutation carriers in this cohort. Out of the 23 patients having a known genetic cause of PD, 7 patients also have an additional rare variant in either DNAH1, STAB1, ANK2, SH3GL2, and/or NOD2 . Two patients with a LRRK2 p.R1441C mutation also carry a DNAH1 p.S1089G variant. Two sisters with the STAB1 p.S1089G variant in our discovery cohort harbor a homozygous PINK1 p.Q456X variant. One patient with a negative FH of PD and an AAO of 35 years is compound heterozygous for pathogenic PRKN variants and also an ANK2 p.V3634D variant. Patient L-649 is a carrier of a heterozygous PRKN p.R275W and an SH3GL2 p.G276V variant, with a positive FH of PD and an AAO of 16 years. Lastly, patient L-1888 had compound heterozygous PRKN variants and a NOD2 p.G908R variant.
Diagnosing Rare Forms Of Parkinsons Disease
Research2015-2017 Research Projects
Positron Emission Tomography Imaging of Pathological Tau in Parkinsonisms
For most people with a classic form of Parkinson’s disease, medication helps control their symptoms. At least two other progressive brain disorders have similar symptoms, though, and the same medication isn’t as effective – but researchers can’t yet distinguish between the diseases.
At the University of Toronto and the Centre for Mental Health and Addiction, neuroscientist Sarah Coakeley is using imaging technology to develop a diagnostic test for progressive supranuclear palsy, or PSP. PSP is one of the rare disorders that affects movement and may initially appear to be Parkinson’s. People with PSP deteriorate more rapidly than people with Parkinson’s, however, and dopamine replacement therapy doesn’t control their symptoms for long.
Coakeley, a Master’s student, uses Positron Imaging Technology to scan the brains of people with Parkinson’s disease, people whose brains are healthy, and people who have already been diagnosed with either PSP or multiple system atrophy, another rare disorder. The people participating in the study are injected with a special radioactive dye that binds to Tau, a protein in the brain that clumps up in brain cells of people with these neurodegenerative diseases.
“It will give them a more accurate prognosis, so they are prepared for this rapid disease progression,” she says.
Search
What Raises Someone’s Risk For Parkinson’s
It’s a complex picture, but you may be more likely to get Parkinson’s based on:
Age. Since it mostly affects people 60 and older, your risk goes up as the years go by.
Family history. If your parent, brother, or sister has it, you’re a little more likely to get it.
Job. Some types of work, like farming or factory jobs, can cause you to have contact with chemicals linked to Parkinson’s.
Race. It shows up more often in white people than other groups.
Serious head injury. If you hit your head hard enough to lose consciousness or forget things as a result of it, you may be more likely to get Parkinson’s later in life.
Gender. Men get it more than women. Doctors aren’t sure why.
Where you live. People in rural areas seem to get it more often, which may be tied to chemicals used in farming.
Clinical Characteristics Of Eopd Patients
Subjects of the present study were 112 EOPD patients and 112 unrelated healthy individuals. The mean age of patients was 44.5 ± 8.3 years , and the mean age of control group was similar , while the mean age at onset of patients was 37 ± 5.91 years . The sex ratio for EOPD and control was the same, with male:female ratio was 54:58. We found that the average stage of Hoehn and Yahr was 2.24, and the mean score of the Unified Parkinson’s Disease Rating Scale part III was 38.28. Thirty-seven of 112 patients, accounting for 33.03%, showed dystonia. For non-motor symptoms, there were 38 patients expressed constipation, 51 patients with urinary dysfunction, 47 patients with hyperhidrosis, and 11 patients reported hallucination.
What Are Atypical Parkinsonian Disorders
Atypical Parkinsonian disorders are progressive diseases that present with some of the signs and symptoms of Parkinson’s disease, but that generally do not respond well to drug treatment with levodopa. They are associated with abnormal protein buildup within brain cells.
The term refers to several conditions, each affecting particular parts of the brain and showing a characteristic course:
- Dementia with Lewy bodies, characterized by an abnormal accumulation of alpha-synuclein protein in brain cells
- Progressive supranuclear palsy, involving tau protein buildup affecting the frontal lobes, brainstem, cerebellum and substantia nigra
- Multiple system atrophy, another synucleinopathy that affects the autonomic nervous system , substantia nigra and at times the cerebellum
- Corticobasal syndrome, a rare tauopathy that typically affects one side of the body more than the other and makes it difficult for patients to see and navigate through space
What Causes Parkinsons Disease Dementia

A chemical messenger in the brain called dopamine helps control and coordinate muscle movement. Over time, Parkinson’s disease destroys the nerve cells that make dopamine.
Without this chemical messenger, the nerve cells can’t properly relay instructions to the body. This causes a loss of muscle function and coordination. Researchers don’t know why these brain cells disappear.
Parkinson’s disease also causes dramatic changes in a part of your brain that controls movement.
Those with Parkinson’s disease often experience motor symptoms as a preliminary sign of the condition. Tremors are one of the most common first symptoms of Parkinson’s disease.
As the disease progresses and spreads in your brain, it can affect the parts of your brain responsible for mental functions, memory, and judgment.
Over time, your brain may not be able to use these areas as efficiently as it once did. As a result, you may begin experiencing symptoms of Parkinson’s disease dementia.
You have an increased risk of developing Parkinson’s disease dementia if:
- you’re a person with a penis
- you’re older
Who Gets Early Onset Parkinsons Disease
About 10%-20% of those diagnosed with Parkinson’s disease are under age 50, and about half of those are diagnosed before age 40. Approximately 60,000 new cases of Parkinson’s are diagnosed each year in the United States, meaning somewhere around 6,000 – 12,000 are young onset patients.
Is it genetic or hereditary?
The cause of Parkinson’s disease is not yet known. However, Parkinson’s disease has appeared across several generations of some families, which could indicate that certain forms of the disease are hereditary or genetic. Many researchers think that Parkinson’s disease may be caused by genetic factors combined with other external factors. The field of genetics is playing an ever greater role in Parkinson’s disease research, and scientists are continually working towards determining the cause or causes of PD.
Replication Association Test Statistics
Subsequent association tests were performed in the International Parkinson’s Disease Genomics Consortium replication cohort for six nominated variants in five genes . Summary statistics were obtained from the exome sequencing data on a PD database query. Each patient carrying variant of interest from these five genes was obtained from the full IPDGC cohort for further association testing. Genetic association testing on specific variants found in the original German discovery cohort was also performed by comparing the 2,859 unrelated individuals from the IPDGC and 24,146 individuals from the non-Finnish European population from gnomAD using Fisher’s exact test. After correcting for multiple testing, p values < 0.0085 were considered statistically significant.
Table 1. Patients with candidate variants in nominated GWAS loci.